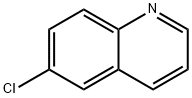
6-CHLOROQUINOLINE synthesis
- Product Name:6-CHLOROQUINOLINE
- CAS Number:612-57-7
- Molecular formula:C9H6ClN
- Molecular Weight:163.6
Yield:612-57-7 86%
Reaction Conditions:
with tetrachloromethane;iron(III) chloride hexahydrate at 150; for 8 h;Inert atmosphere;Sealed tube;
Steps:
General procedure for the preparation of quinolines 2a-2l.
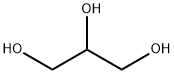
General procedure: An ampule was charged with 0.02 mmol of FeCl3·6H2O, 2 mmol of aniline, 4 mmol of carbon tetrachloride, and 8 mmol 1,3-propanediol under argon. The sealed ampule was placed into a pressure reactor, which was hermetically closed and heated at 150°C for 8 h with continuous stirring. After the reaction completion, the reactor was cooled to room temperature, the ampule was opened, the reaction mixture was poured in hydrochloric acid. The aqueous layer was separated, neutralized with 10% sodium hydroxide solution, and extracted with methylenechloride. The organic layer was filtered, the solvent was distilled off, and the residue was distilled in a vacuum. Physicochemical characteristics and spectral data of the obtained compounds 2a-2l corresponded to the literature data.
References:
Khusnutdinov;Bayguzina;Aminov [Russian Journal of General Chemistry,2015,vol. 85,# 12,p. 2725 - 2727][Zh. Obshch. Khim.,2015,vol. 85,# 12,p. 1993 - 1995,3]

49716-18-9
92 suppliers
$42.00/100mg

612-57-7
222 suppliers
$6.00/1g
6563-10-6
3 suppliers
inquiry

612-57-7
222 suppliers
$6.00/1g