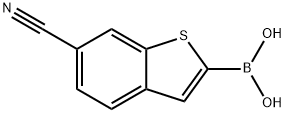
6-cyanobenzo[b]thiophen-2-ylboronic acid synthesis
- Product Name:6-cyanobenzo[b]thiophen-2-ylboronic acid
- CAS Number:1119899-35-2
- Molecular formula:C9H6BNO2S
- Molecular Weight:203.03
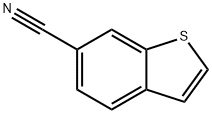
154650-81-4
35 suppliers
inquiry
![6-cyanobenzo[b]thiophen-2-ylboronic acid](/CAS/GIF/1119899-35-2.gif)
1119899-35-2
12 suppliers
inquiry
Yield:1119899-35-2 72%
Reaction Conditions:
Stage #1: benzo[b]thiophene-6-carbonitrilewith n-butyllithium in tetrahydrofuran;hexanes at -90; for 0.5 h;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexanes at -90; for 0.5 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexanes at -90 - 30; for 0.5 h;
Steps:
35
[0167] Compound 23 (14 g, 0.088 mol) in dry THF (350 mL) was purged with nitrogen. The mixture was then cooled (-90 0C) with liquid nitrogen bath and ra-BuLi (2.5 M in hexanes,
References:
WO2009/26345,2009,A1 Location in patent:Page/Page column 43-44