
6-Ethylpicolinonitrile synthesis
- Product Name:6-Ethylpicolinonitrile
- CAS Number:59146-66-6
- Molecular formula:C8H8N2
- Molecular Weight:132.16
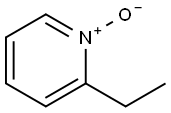
4833-24-3
21 suppliers
$45.00/100mg
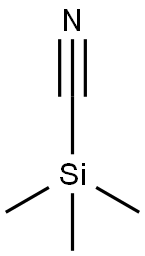
7677-24-9
373 suppliers
$19.00/5g

59146-66-6
20 suppliers
inquiry
Yield:59146-66-6 31%
Reaction Conditions:
with N,N-Dimethylcarbamoyl chloride in Nitroethane at 20; for 72 h;
Steps:
39 Reference Example 39
Reference Example 39 2-Cyano-6-ethylpyridine 2-Ethylpyridine N-oxide (3.0 g, 24.4 mmol) was dissolved in nitroethane (30 ml), and trimethylsilyl cyanide (2.4 g, 24.0 mmol) AndN,N-dimethylcarbamoyl chloride (2.4 g, 21.9 mmol) were added thereto.. The reaction mixture was stirred at room temperature for 3 days, concentrated under reduced pressure, combined with saturated aqueous sodium hydrogen carbonate solution and extracted with ethyl acetate.. The extract was washed with saturated brine and dried.. The solvent was evaporated under reduced pressure.. The residue was subjected to a silica gel column chromatography and eluted with hexane-ethyl acetate (2:1, v/v) to give the titled compound (1.0, 31 %).1H-NMR (CDCl3) δ: 1.32 (3H, t, J = 7.5 Hz), 2.87 (2H, q, J = 7.5 Hz), 7.38 (1H, d, J = 7.8 Hz), 7.52 (1H, d, J = 7.5 Hz), 7.73 (1H, t, J = 7.8 Hz).
References:
EP1424336,2004,A1 Location in patent:Page 108
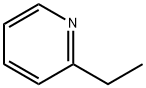
100-71-0
179 suppliers
$10.00/5g

59146-66-6
20 suppliers
inquiry
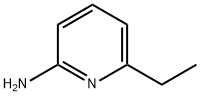
21717-29-3
77 suppliers
$15.00/1g

59146-66-6
20 suppliers
inquiry
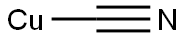
544-92-3
247 suppliers
$9.00/1g

83004-13-1
46 suppliers
$83.00/100mg

59146-66-6
20 suppliers
inquiry