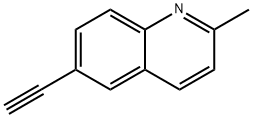
6-ethynyl-2-methylquinoline synthesis
- Product Name:6-ethynyl-2-methylquinoline
- CAS Number:1233505-71-9
- Molecular formula:C12H9N
- Molecular Weight:167.21
![2-methyl-6-[2-(trimethylsilyl)ethynyl]quinoline](/CAS/20210111/GIF/1315248-52-2.gif)
1315248-52-2
4 suppliers
inquiry

1233505-71-9
7 suppliers
inquiry
Yield:1233505-71-9 98%
Reaction Conditions:
with methanol;potassium carbonate at 20;
Steps:
C-6
6) Preparation of 6-ethynyl-2-methyl-quinolineTo a solution of 2-methyl-6-trimethylsilanylethynyl-quinoline (2.1 g, 1.03 mmol,1 eq) in solution in MeOH (5 mL) was added K2C03 (5.69 g, 4.12 mmol, 4 eq), and the resulting mixture was stirred overnight at room temperature. The solvent was then removed under reduced pressure and water was then added. The aqueous layer was extracted twice with cyclohexane, and then the combined organic layers were washed with brine and dried over MgS04. After filtration and concentration under reduced pressure the crude product was purified by column chromatography on silica gel (Cyclohexane-EtOAc 99:1) to afford 169 mg of 6-ethynyl-2-methyl-quinoline as a yellow crystal (98%).Molecular formula: Ci2H9NMolecular weight: 167.21 g.mol"1 NMR (500 MHz): £ 7.94 (d, J = 8.5 Hz, 1H, ?), 7.93 (d, J = 8.0 Hz, 1H, ?), 7.90 (d, J= 1.0 Hz, 1H, H5), 7.70 (dd, J= 8.5 Hz, J= 1.0 Hz, 1H, H7), 7.25 (d, J= 8.0 Hz, 1H, H3), 3.16 (s, 1H, H?), 2.72 (s, 3H, H9).13C NMR (125 MHz): £ 161.4 (s, C2), 148.8 (s, C8a), 137.2 (s, C4), 133.7 (s, C7), 133.1 (s, C5), 130.3 (s, C8), 127.4 (s, C4a), 124.1 (s, C3), 120.8 (s, C6), 84.7 (s, C10), 79.4 (s, Cn), 26.8 (s, C9).
References:
WO2011/86469,2011,A1 Location in patent:Page/Page column 40
877-42-9
188 suppliers
$16.00/1g

1233505-71-9
7 suppliers
inquiry

106-40-1
458 suppliers
$12.00/5g

1233505-71-9
7 suppliers
inquiry