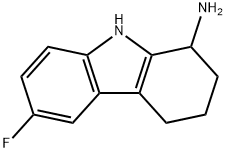
6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-1-amine synthesis
- Product Name:6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-1-amine
- CAS Number:1429901-83-6
- Molecular formula:C12H13FN2
- Molecular Weight:204.24
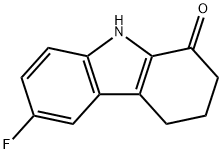
41734-98-9
15 suppliers
inquiry

1429901-83-6
14 suppliers
inquiry
Yield:1429901-83-6 30%
Reaction Conditions:
with ammonium acetate;sodium cyanoborohydride in methanol at 60;
Steps:
12 6-Fluoro-2,3 ,4,9-tetrahydro- 1H-carbazol- 1-amine
6-Fluoro-2, 3,4, 9-tetrahydro- 1H-carb azol- 1-one (400 mg, 1.97 mmol), ammonium acetate (1.52 g, 19.7 mmol) and sodium cyanoborohydride (619 mg, 9.85 mmol) were dissolved in methanol (10 mL). The reaction mixture was stirred for overnight at 60 °C. After removal of solvent, the reaction mixture was diluted with ethyl acetate. The organic layer was washed with 10% sodium hydroxide and brine, and it was dried over sodium sulfate. The organic layer was concentrated under reduced pressure. The residue was purified on an ISCO chromatograph (0-10% methanol/dichloromethane + 0.1% NH4OH) to give the product as a white solid (120 mg,30%);’H NIVIR (300 IVIHz) (DMSO-d6) 10.76 (bs, 1H), 7.24 (dd, J= 9 Hz, J= 5 Hz, 1H), 7.06(d, J 10Hz, 1H), 6.83-6.77 (m, 1H), 3.90 (m, 1H), 1.97 (m, 4H), 1.73-1.46 (m, 2H); LC/IVISRT = 2.46 (M-H: 203).
References:
WO2019/5841,2019,A1 Location in patent:Page/Page column 82; 83
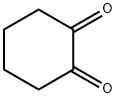
765-87-7
322 suppliers
$6.00/1g

1429901-83-6
14 suppliers
inquiry
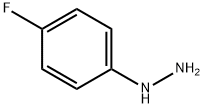
371-14-2
80 suppliers
inquiry

1429901-83-6
14 suppliers
inquiry