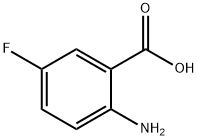
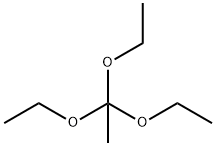
6-fluoro-2-methyl-4H-benzo[d][1,3]oxazin-4-one synthesis
- Product Name:6-fluoro-2-methyl-4H-benzo[d][1,3]oxazin-4-one
- CAS Number:38520-78-4
- Molecular formula:C9H6FNO2
- Molecular Weight:179.15
201230-82-2
1 suppliers
inquiry
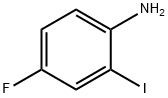
61272-76-2
189 suppliers
$9.00/5g

108-24-7
5 suppliers
$14.00/250ML
![6-fluoro-2-methyl-4H-benzo[d][1,3]oxazin-4-one](/CAS/GIF/38520-78-4.gif)
38520-78-4
21 suppliers
inquiry
Yield:38520-78-4 84%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in toluene at 100; under 1500.15 Torr; for 24 h;Inert atmosphere;Autoclave;
Steps:
General procedure for the carbonylative synthesis of 2-alkylbenzoxazinones underthe catalysis of heterogeneous palladium
General procedure: A 12mL vial equipped with a septum, a small cannula, and a magnetic stirring bar wascharged with 2 P-MCM-41-PdCl2 (0.02 mmol) and 2-iodoaniline (1 mmol). After thevial was purged with argon, acid anhydride (1.5 mmol), DiPEA (2.2 mmol), and drytoluene (2 mL) were injected into the vial by syringe. Then the vial was placed in analloy plate, which was transferred into an autoclave (300 mL) under Ar. After the autoclavebeing flushed with carbon monoxide three times, the pressure of carbon monoxidewas adjusted to 2 bar, and the reaction mixture was stirred at 100 C for 24 h. After theautoclave being cooled to ambient temperature, the CO pressure was cautiouslyreleased. The reaction mixture was diluted with EtOAc (10 mL) and filtered. The catalystwas washed with deionized water (24 mL) and acetone (24 mL), dried at 80 Cin vacuo for 2 h, and reused directly in the next run. The filtrate was washed with water(26 mL), dried over Na2SO4, and evaporated in vacuo. The crude product was purifiedby silica gel column chromatography (light petroleum-ethyl acetate 7:3) todeliver the desired products 3.
References:
Zhou, Zebiao;Huang, Bin;Cai, Mingzhong [Synthetic Communications,2021,vol. 51,# 20,p. 3150 - 3163]
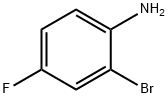
1003-98-1
393 suppliers
$5.00/5g
![6-fluoro-2-methyl-4H-benzo[d][1,3]oxazin-4-one](/CAS/GIF/38520-78-4.gif)
38520-78-4
21 suppliers
inquiry
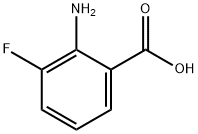
825-22-9
340 suppliers
$13.00/5g
![6-fluoro-2-methyl-4H-benzo[d][1,3]oxazin-4-one](/CAS/GIF/38520-78-4.gif)
38520-78-4
21 suppliers
inquiry