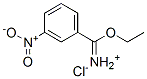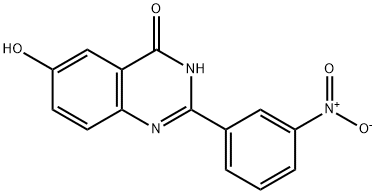
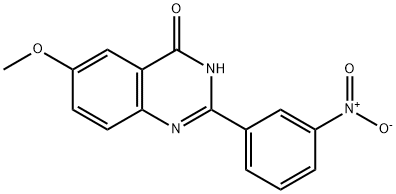
6-hydroxy-2-(3-nitrophenyl)quinazolin-4(3H)-one synthesis
- Product Name:6-hydroxy-2-(3-nitrophenyl)quinazolin-4(3H)-one
- CAS Number:371946-32-6
- Molecular formula:C14H9N3O4
- Molecular Weight:283.24
911418-31-0
0 suppliers
inquiry

371946-32-6
5 suppliers
inquiry
Yield:371946-32-6 80%
Reaction Conditions:
with boron tribromide in dichloromethane at -78 - 20;
Steps:
131
To a suspension of 6-methoxy-2-(3-nitrophenyl)quinazolin-4(3H)-one (3.9Og, 13.1 mmol), in CH2Cl2 (30 mL) cooled to -78 °C under an atmosphere of N2 was added BBr3 as a 1.0M solution in CH2Cl2 (20 mL, 20.0 mmol). The resulting mixture was stirred at -78 °C for 1 h, then allowed to warm to RT upon which it was stirred for a further 3 h. The mixture was re-cooled to -78 °C and stirred overnight. The reaction was quenched by the addition of EtOH (60 mL) and allowed to warm to RT. Stirring was continued for 1 h at RT, upon which a precipitate formed. Sat. NaHCO3 solution was added and the yellow solid was collected via filtration and washed with Et2O and EtOH and dried under vacuum to give 6-hydroxy-2-(3-nitrophenyl)quinazolin-4(3H)-one (2.96g, 10.5 mmol, 80%). HPLC retention time 5.588 min.
References:
WO2008/54599,2008,A2 Location in patent:Page/Page column 174

1882-69-5
289 suppliers
$6.00/5g

371946-32-6
5 suppliers
inquiry

41994-92-7
11 suppliers
$45.00/100mg

371946-32-6
5 suppliers
inquiry

63932-00-3
0 suppliers
inquiry

371946-32-6
5 suppliers
inquiry