6-Hydroxy-2-methylchromone synthesis
- Product Name:6-Hydroxy-2-methylchromone
- CAS Number:22105-12-0
- Molecular formula:C10H8O3
- Molecular Weight:176.17
![Ethanone, 1-[2,5-bis(acetyloxy)phenyl]-](/CAS/20210305/GIF/31405-72-8.gif)
31405-72-8
0 suppliers
inquiry

22105-12-0
6 suppliers
inquiry
Yield:22105-12-0 46%
Reaction Conditions:
Stage #1: 2,5-diacetoxyacetophenonewith potassium tert-butylate in tetrahydrofuran;
Stage #2: with sodium hydroxide;copper dichloride in tetrahydrofuran at 20; for 24 h;
Steps:
2.a Example 2: Synthesis of Compound of Formula (2) (2-Methyl-6-Chromanol) (see FIG. 3)
First step a): 1) KOtBu, tetrahydrofuran; 2) CuCl2; 3) NaOH; 24 hours at room temperature; yield: 46%.Second step b): H2, Pd/C; EtOH; 70° C., 7 days, 10 bar, yield: 20%.Starting from 2-acetyl-1,4-phenylene diacetate a base-mediated intramolecular aldol condensation furnishes the enone intermediate as described before (A. O. Termath, Stereoselektive Totalsynthese von a-Tocopherol durch Cu-katalysierte asymmetrische 1,4-Addition an ein Chromenon, Dissertation, Universit?t zu K?ln, ISBN 978-3-8439-1407-9, Verlag Dr. Hut, München, 2013; Chapter 6.2.2, page 196-197.). The subsequent hydrogenation, using similar conditions as for compound of formula (1) above, furnishes the product in 20% yield.
References:
US2021/30022,2021,A1 Location in patent:Paragraph 0174-0176
![Ethanone, 1-[2,5-bis(benzoyloxy)phenyl]-](/CAS/20210305/GIF/76323-08-5.gif)
76323-08-5
0 suppliers
inquiry

22105-12-0
6 suppliers
inquiry
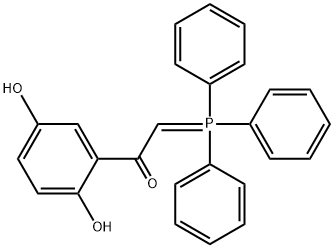
![Phosphonium, [2-[2,5-bis(benzoyloxy)phenyl]-2-oxoethyl]triphenyl-, bromide (1:1)](/CAS/20211123/GIF/107139-52-6.gif)
107139-52-6
0 suppliers
inquiry

22105-12-0
6 suppliers
inquiry
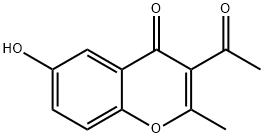
401515-47-7
2 suppliers
inquiry

22105-12-0
6 suppliers
inquiry