
6-HYDROXYCAPROIC ACID synthesis
- Product Name:6-HYDROXYCAPROIC ACID
- CAS Number:1191-25-9
- Molecular formula:C6H12O3
- Molecular Weight:132.16

108-94-1
528 suppliers
$12.00/50g
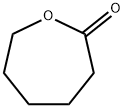
502-44-3
337 suppliers
$5.00/25g

1191-25-9
113 suppliers
$14.14/250mgs:
Yield:1191-25-9 85.4% ,502-44-3 8.3%
Reaction Conditions:
with dihydrogen peroxide;acetic acid in water at 70; for 1 h;Reagent/catalyst;
Steps:
1; 2; 1 Example 1
Stirrer chip in a glass container, cyclohexanone 0.982 g (10 mmol), beta zeolite 0.1 g, 30 mass% hydrogen peroxide 1.36 g (12 mmol), acetic acid 0.006 g (0.1 mmol), water 6 g were added and stirred at 70 ° C. for 1 hour.Then, the reaction liquid was cooled to room temperature, and the insoluble matter was removed by filtration.Next, when acetonitrile and t-butyl alcohol were added to the obtained filtrate to make the liquid phase one phase and then analyzed by high performance liquid chromatography (HPLC), the yield of 6-hydroxycaproic acid was 85.4% and the yield of caprolactone was 8.3%.The maximum amount of peracid that can be generated during the reaction depends on the amount of acetic acid added, and was 0.71% by mass or less in this example.This is far below the lower limit of 21 mass% at which peracetic acid exhibits explosive properties.In addition, no dangerous phenomena such as explosion and rapid heat generation were observed during the reaction.The yields of 6-hydroxycaproic acid and caprolactone were measured by an internal standard method using liquid chromatography.The analysis conditions are shown below.
References:
Asahi Kasei Corporation;Tsuji, Yuki;Urayama, Teppei;Miyazaki, Ryusuke JP2020/59682, 2020, A Location in patent:Paragraph 0025-0027

108-94-1
528 suppliers
$12.00/50g

1191-25-9
113 suppliers
$14.14/250mgs:
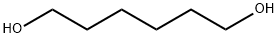
629-11-8
442 suppliers
$6.00/25g

1191-25-9
113 suppliers
$14.14/250mgs:

502-44-3
337 suppliers
$5.00/25g

1191-25-9
113 suppliers
$14.14/250mgs:
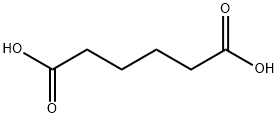
124-04-9
995 suppliers
$6.00/25g

502-44-3
337 suppliers
$5.00/25g

1191-25-9
113 suppliers
$14.14/250mgs:

120-92-3
399 suppliers
$11.00/25g