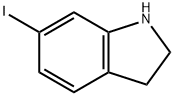
6-IODO-2,3-DIHYDRO-1H-INDOLE HYDROCHLORIDE synthesis
- Product Name:6-IODO-2,3-DIHYDRO-1H-INDOLE HYDROCHLORIDE
- CAS Number:115666-46-1
- Molecular formula:C8H8IN
- Molecular Weight:245.06
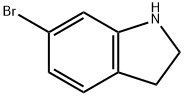
63839-24-7
135 suppliers
$16.00/100mg

115666-46-1
61 suppliers
$34.00/100mg
Yield: 83%
Reaction Conditions:
with copper(l) iodide;lithium iodide;N,N`-dimethylethylenediamine in 1,4-dioxane at 110; for 24 h;Inert atmosphere;Sealed tube;
Steps:
6-iodoindoline (51)
Compound 51 was synthesized using a slightly modified protocol published by Klapars and Buchwald.3 An oven or flame dried, microwave glass tube was charged with a magnetic stir bar, compound 50 (1.0 g, 5.05 mmol), Cul (106 mg, 0.555 mmol), and Lil (1.49 g, 11.11 mmol). The glass tube was evacuated under vacuum and backfilled with N2 5 times, DMEDA (120 pL) was added into the reaction vessel quickly before the tube was sealed with a Teflon cap. Anhydrous l,4-dioxane (5 mL) was delivered via a syringe. The reaction was then heated to 110° C and stirred for 24 h. After cooling to rt, the reaction mixture was diluted with 10 mL sat. NELCl solution and extracted with DCM (4 x 25 mL). The combined organic layers were washed with brine and dried over anhydrous Na2S04, then concentrated in vacuo. The residue was purified by flash column chromatography with silica gel, using DCM/Hexane as eluent to give compound 51 (1.03 g, 83%).
References:
OREGON HEALTH & SCIENCE UNIVERSITY;GIBBS, Summer, L.;WANG, Lei, G.;BARTH, Connor, W. WO2020/56046, 2020, A1 Location in patent:Page/Page column 50

115666-47-2
105 suppliers
$32.00/100mg

115666-46-1
61 suppliers
$34.00/100mg
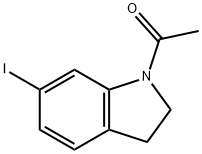
115666-43-8
6 suppliers
inquiry

115666-46-1
61 suppliers
$34.00/100mg
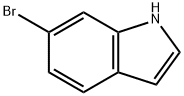
52415-29-9
413 suppliers
$9.00/1g

115666-46-1
61 suppliers
$34.00/100mg

41252-97-5
181 suppliers
$6.00/1g

115666-46-1
61 suppliers
$34.00/100mg