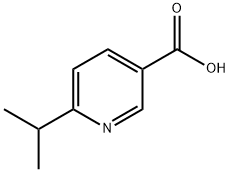
6-Isopropylnicotinic acid synthesis
- Product Name:6-Isopropylnicotinic acid
- CAS Number:886214-81-9
- Molecular formula:C9H11NO2
- Molecular Weight:165.19

166331-65-3
21 suppliers
inquiry

886214-81-9
17 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:2-chloro-6-isopropylnicotinic acid with hydrogen;palladium 10% on activated carbon in ethanol;water at 20; for 72 h;
Stage #2: with sodium hydroxide in water
Steps:
10
Preparation Example 10 2-Chloro-6-isopropylnicotinic acid (312 g), palladium carbon(10%) (16.0 g) and ethanol-water (6:1) (2000 mL) were stirred under a hydrogen atmosphere at room temperature for 3 days. The reaction solution was filtrated, and the filtrate was concentrated under reduced pressure. Ethyl acetate and a small amount of ethanol were added to the residue, and the precipitated solid was collected by filtration. The solid was added to cooled 1N-aqueous sodium hydroxide solution (1130 mL) and, after dissolution, the precipitated solid was collected by filtration. This was dissolved in chloroform, and dried over magnesium sulfate. The solvent was evaporated, the residual solid was suspended in hexane and collected by filtration to give 6-isopropylnicotinic acid (152 g) as white powder crystals. 1H-NMR(CDCl3)δ: 1.37(6H,d,J=6.9Hz), 3.29(1H,sept,J=6.9Hz), 7.38(1H,d,J=8.1Hz), 8.40(1H,dd,J=2.1,8.1Hz), 9.33(1H,d,J=2.1Hz).
References:
Mitsubishi Pharma Corporation EP1852431, 2007, A1 Location in patent:Page/Page column 28
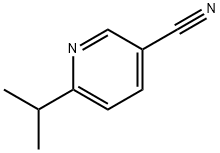
1033750-40-1
15 suppliers
inquiry

886214-81-9
17 suppliers
inquiry