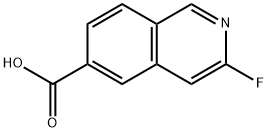
6-Isoquinolinecarboxylic acid, 3-fluoro- synthesis
- Product Name:6-Isoquinolinecarboxylic acid, 3-fluoro-
- CAS Number:1104071-70-6
- Molecular formula:C10H6FNO2
- Molecular Weight:191.16
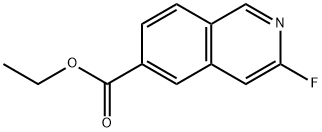
1104071-68-2
0 suppliers
inquiry

1104071-70-6
8 suppliers
inquiry
Yield:1104071-70-6 100%
Reaction Conditions:
Stage #1: ethyl 3-fluoroisoquinoline-6-carboxylatewith lithium hydroxide;water in tetrahydrofuran;methanol at 20; for 3 h;
Stage #2: with citric acid in water; pH=4;
Steps:
43
3-Fluoroisoquinoline-6-carboxylic acid. A mixture of methyl 3- fluoroisoquinoline-6-carboxylate (0.257 g, 1.25 mmol) and lithium hydroxide, monohydrate (105 mg, 2.51 mmol) in THF:MeOH:H2O (3:2: 1, 4 mL) was stirred at room temperature for 3 hours. The MeOH and THF were evaporated. 5 % citric acid was added, and the mixture was futher acidified to pH = 4 with NaHSO4. The precipitate was recovered by filtration. The filtrate was extracted with chlrorform-isopropanol. The organic extracts were washed with water, brine, dried over sodium sulfate and evaporated. The solids were combined to provide the product as a white solid (1.12 g, 100 %). LCMS (API-ES) m/z: 192 (M+H+).
References:
WO2009/11871,2009,A2 Location in patent:Page/Page column 188; 190
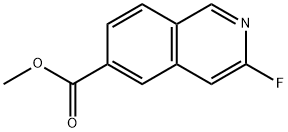
1241675-07-9
10 suppliers
inquiry

1104071-70-6
8 suppliers
inquiry