
6-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline synthesis
- Product Name:6-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline
- CAS Number:54893-54-8
- Molecular formula:C11H15NO
- Molecular Weight:177.24

50-00-0
896 suppliers
$10.00/25g
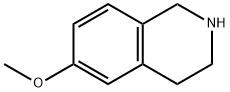
42923-77-3
144 suppliers
$21.00/100mg

54893-54-8
20 suppliers
inquiry
Yield:54893-54-8 260 g
Reaction Conditions:
Stage #1: formalin;6-methoxy-1,2,3,4-tetrahydro-isoquinoline in methanol at 0; for 0.25 h;Inert atmosphere;
Stage #2: with sodium tetrahydridoborate in methanol at 0; for 3 h;Inert atmosphere;
Steps:
3 Step 3.
A 5-L 4-necked round-bottom flask was charged with 6-methoxy-1,2,3,4- tetrahydroisoquinoline (370 g, 2.27 mol), MeOH (4.0 L) and formaldehyde (1.1 L) was added slowly at 0 oC. The resulting solution was stirred for 15 min, then NaBH4 (300 g, 7.93 mol) was added in portions at 0 oC. The resulting solution was stirred for 3 h, and quenched with water (1 L). The resulting mixture was stirred for 30 min, then concentrated under reduced pressure. The resulting mixture was diluted with water (5 L) and extracted with DCM (3 x 3 L). The combined organic layers were concentrated in vacuo, giving 6-methoxy-2-methyl- 1,2,3,4-tetrahydroisoquinoline (260 g).
References:
WO2022/98809,2022,A1 Location in patent:Page/Page column 51; 54-55

50-00-0
896 suppliers
$10.00/25g
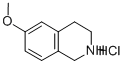
57196-62-0
140 suppliers
$9.00/250mg

54893-54-8
20 suppliers
inquiry
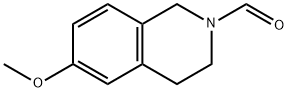
212188-99-3
0 suppliers
inquiry

54893-54-8
20 suppliers
inquiry

2039-67-0
241 suppliers
$17.46/5G

54893-54-8
20 suppliers
inquiry
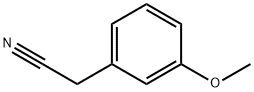
19924-43-7
271 suppliers
$9.00/10g

54893-54-8
20 suppliers
inquiry