6-Methoxy-2-phenylimidazo[1,2-a]pyridine synthesis
- Product Name:6-Methoxy-2-phenylimidazo[1,2-a]pyridine
- CAS Number:869583-76-6
- Molecular formula:C14H12N2O
- Molecular Weight:224.26
Yield:869583-76-6 80%
Reaction Conditions:
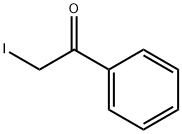
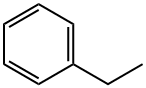
Stage #1: ethylbenzenewith N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in water;ethyl acetate at 65; for 1.5 h;
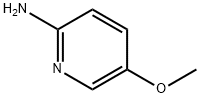
Stage #2: 2-amino-5-methoxypyridinewith sodium carbonate in water at 80;
Steps:
3.2.1. General Procedure for the Synthesis of Nitrogen-Containing Heterocycles
General procedure: Synthesis of 3a is representative. To a solution of ethylbenzene (1a, 1 mmol, 107 mg) in ethylacetate:water (5:1, 6 mL) were added NBS (3.5 mmol, 628 mg) and AIBN (0.1 mmol, 16.5 mg) at roomtemperature, and the mixture was stirred at 65 °C for 1.5 h. The mixture was concentrated to drynessand then dissolved in water (5 mL), followed by reaction with 2-aminopyridine (2a, 1.2 mmol, 114 mg)and sodium carbonate (5 mmol, 534 mg) for 2 h at 80 °C. After completion of the reaction (as indicatedby TLC), the crude product was extracted with ethyl acetate (3 x 10 mL). The combined organic layerwas dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified bysilica gel column chromatography (PE/EA = 8/1-4/1, v/v) to give 3a (78% yield) as a white solid.
References:
Chen, Liang;Zhu, Huajian;Wang, Jiang;Liu, Hong [Molecules,2019,vol. 24,# 5,art. no. 893]
10167-97-2
219 suppliers
$18.00/1g

70-11-1
287 suppliers
$10.00/25g
![6-Methoxy-2-phenylimidazo[1,2-a]pyridine](/CAS2/GIF/869583-76-6.gif)
869583-76-6
17 suppliers
inquiry
100-41-4
334 suppliers
$5.00/5G
![6-Methoxy-2-phenylimidazo[1,2-a]pyridine](/CAS2/GIF/869583-76-6.gif)
869583-76-6
17 suppliers
inquiry

![6-BROMO-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/4044-98-8.gif)