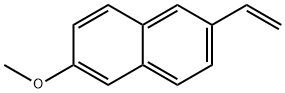
6-Methoxy-2-vinylnaphthalene synthesis
- Product Name:6-Methoxy-2-vinylnaphthalene
- CAS Number:63444-51-9
- Molecular formula:C13H12O
- Molecular Weight:184.23
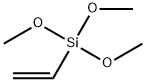
2768-02-7
386 suppliers
$10.00/5g
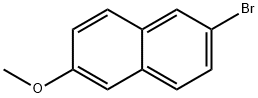
5111-65-9
339 suppliers
$6.00/5g

63444-51-9
118 suppliers
$135.00/1g
Yield: 84%
Reaction Conditions:
with (1,2-dimethoxyethane)dichloronickel(II);18-crown-6 ether;potassium methanolate in N,N-dimethyl-formamide at 35; for 12 h;Inert atmosphere;Hiyama Coupling;Reagent/catalyst;Solvent;
Steps:
General Procedure 1
General procedure: In a nitrogen-filled glove box, aromatic halide (0.2 mmol, 1.0equiv), 18-crown-6 (121.0 mg, 0.46 mmol, 2.3 equiv), KOMe(32.2 mg, 0.46 mmol, 2.3 equiv), NiCl2(glyme) (4.4 mg, 0.02mmol, 10 mol%), and DMF (1.0 mL) were charged to an 8 mL vialequipped with a magnetic stirrer bar. The vinyltrimethoxysilane(68.0 mg, 0.46 mmol, 2.3 equiv) was added. The vial wasremoved from the glove box, and the reaction mixture wasstirred at rt (35 °C) for 12 h. The reaction mixture was thendiluted with EtOAc and washed with water. The organic phasewas dried over Na2SO4, filtered, and concentrated, and theresidue was purified by column chromatography on silica gel togive the product.2-Methoxy-6-vinylnaphthalene (3a)Using general procedure 1: white solid, 31.0 mg, yield: 84%. 1HNMR (400 MHz, CDCl3): = 7.78-7.66 (m, 3 H), 7.62 (dd, J = 8.7,1.7 Hz, 1 H), 7.19-7.10 (m, 2 H), 6.87 (dd, J = 17.6, 10.9 Hz, 1 H),5.84 (dd, J = 17.6, 0.9 Hz, 1 H), 5.30 (dd, J = 10.9, 0.9 Hz, 1 H),3.93 (s, 3 H).
References:
Wei, Shichao;Mao, Yongjun;Shi, Shi-Liang [Synlett,2021,art. no. ST-2020-K0612-C] Location in patent:supporting information
593-60-2
136 suppliers
$25.00/25g

5111-65-9
339 suppliers
$6.00/5g

63444-51-9
118 suppliers
$135.00/1g
74-85-1
96 suppliers
$79.00/11l

5111-65-9
339 suppliers
$6.00/5g

63444-51-9
118 suppliers
$135.00/1g

129113-00-4
40 suppliers
$41.10/1g

63444-51-9
118 suppliers
$135.00/1g

2627-95-4
322 suppliers
$16.80/25G

5111-65-9
339 suppliers
$6.00/5g

63444-51-9
118 suppliers
$135.00/1g