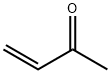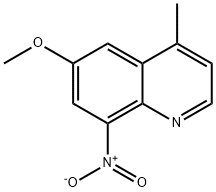
6-METHOXY-4-METHYL-8-NITRO-QUINOLINE synthesis
- Product Name:6-METHOXY-4-METHYL-8-NITRO-QUINOLINE
- CAS Number:64992-56-9
- Molecular formula:C11H10N2O3
- Molecular Weight:218.21
Yield:64992-56-9 87%
Reaction Conditions:
with phosphoric acid;orthoarsenic acid at 120; for 0.5 h;
Steps:
General procedure: To a mixture of 0.24 g (0.86 mmol) of 4-amino-2-(3-fluorophenyloxy)-5-nitroanisole and 0.40 g (1.74 mmol) of H3AsO4 in 3 mL of 85 % H3PO4 at 120 °C, 91 mg (1.3 mmol) of 3-buten-2-one was added dropwise. After stirring for 30 min, the dark solution was poured onto a mixture ofice and water, basified to pH ~ 10 with 2N NaOH, and extracted three times with dichloromethane. The combined extract was washed with water and brine, dried (MgSO4),concentrated, and column chromatographed on silica gel using a mixture of hexane and ethyl acetate (2:1) as an eluent to give 99 mg (35% yield) of compound 31 as a brown solid.
References:
Lu, Jianyu;Maezawa, Izumi;Weerasekara, Sahani;Erenler, Ramazan;Nguyen, Tuyen D.T.;Nguyen, James;Swisher, Luxi Z.;Li, Jun;Jin, Lee-Way;Ranjan, Alok;Srivastava, Sanjay K.;Hua, Duy H. [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 15,p. 3392 - 3397] Location in patent:supporting information
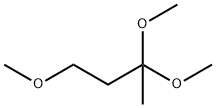
6607-66-5
18 suppliers
$233.00/25g
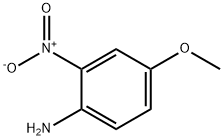
96-96-8
10 suppliers
$7.00/1g

64992-56-9
12 suppliers
$172.00/50MG