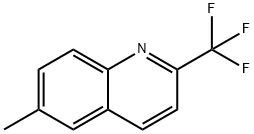
6-METHYL-2-(TRIFLUOROMETHYL)QUINOLINE synthesis
- Product Name:6-METHYL-2-(TRIFLUOROMETHYL)QUINOLINE
- CAS Number:1860-47-5
- Molecular formula:C11H8F3N
- Molecular Weight:211.18
![Benzenamine, N-[(2E)-4,4,4-trifluoro-3-phenoxy-2-buten-1-ylidene]-, [N(E)]-](/CAS/20211123/GIF/1548899-28-0.gif)
1548899-28-0
0 suppliers
inquiry
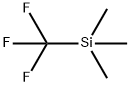
106-49-0
390 suppliers
$5.00/5G
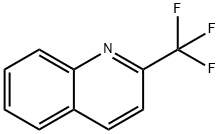
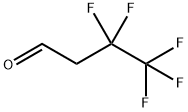
347-42-2
133 suppliers
$20.00/100mg

1860-47-5
24 suppliers
inquiry
Yield:1860-47-5 0.498 g ,347-42-2 75.8 mg
Reaction Conditions:
in dichloromethane at 40; for 6 h;Overall yield = 80 %;
Steps:
2 5.7
Reactivity study of 5a-g: general procedure for the preparation of substituted 2-trifluoromethyl and 2-perfluoroalkylquinolines 6a-h
General procedure: In a typical procedure, a mixture of the imino-enol-ether derivatives 5a-g (1 equiv) and substituted arylamines ( 1equiv) in dichloromethane (10 mL/1 g of 5) was refluxed at 40 °C until disappearance of 19F NMR signals corresponding to the starting products 5 (6-24 h).
The reaction mixture was concentrated under reduced pressure to give brown oil.
Chromatography on silica gel column (eluent: petroleum ether/ethyl acetate 98:2) left two yellow oils, which were precipitated from methanol/water to give pure samples of the corresponding substituted quinolines 6a-h as amorphous white solids.
Formation of the corresponding diazapentadiene intermediates was detected by mass spectrometry showing three different picks corresponding to the molecular mass [M+H]+ of 7 after 2 h of heating.
5.7.1
Reaction of 5a with 4-methylaniline: synthesis of 2-trifluoromethylquinoline (6a) and 2-trifluoromethyl-6-methylquinoline (6b).
Starting with 5a (1 g; 3.43 mmol) and 4-methylaniline (0.367 g; 3.43 mmol) in 10 mL of dichloromethane under reflux for 6 h, 75.8 mg of 2-trifluoromethylquinoline 6a (14%) and 0.498 g of 2-trifluoromethyl-6-methylquinoline 6b (86%) are obtained, total yield 80%.
References:
El Kharrat, Salem;Laurent, Philippe;Blancou, Hubert [Tetrahedron,2014,vol. 70,# 6,p. 1252 - 1266]
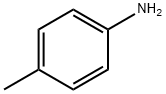
18706-27-9
28 suppliers
$115.00/1g

1860-47-5
24 suppliers
inquiry
![3-Buten-2-one, 1,1,1-trifluoro-4-[(4-methylphenyl)amino]-, (3Z)-](/CAS/20200515/GIF/512778-41-5.gif)
512778-41-5
0 suppliers
inquiry

1860-47-5
24 suppliers
inquiry