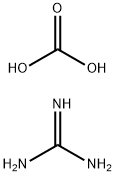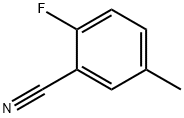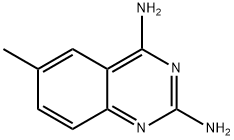
6-Methyl-quinazoline-2,4-diamine synthesis
- Product Name:6-Methyl-quinazoline-2,4-diamine
- CAS Number:1955-61-9
- Molecular formula:C9H10N4
- Molecular Weight:174.2
Yield: 54%
Reaction Conditions:
in N,N-dimethyl acetamide at 140; for 4 h;Inert atmosphere;
Steps:
2,4-Diamino-6-methylquinazoline (20):
0.500 g of 2-fluoro-5-methylbenzonitrile 41 (3.70 mmol) and 1.00 g (5.60 mmol) of guanidine carbonate were dissolved in 5 ml of abs. N,N-dimethylacetamide and heated to 140 °C for 4 h under argon. After cooling a white solid precipitated. The mixture was stored for 18 h in the refrigerator and the product was separated by filtration. After recrystallization from water 350 mg (54 %) of 20 were obtained as colorless crystals. Mp.: 255 °C (lit.255.5-256.6 °C [14]). 1H-NMR(250 MHz, DMSO-d6): / ppm = 7.76 (s, 1H, Ar-H),7.37-7.26 (dd,J =8.5 Hz,J =1.8 Hz, 1H, Ar-H),7.14 (br, 2H, NH2),(d,J =8.5 Hz, 1H, Ar-H),5.84 (s, 2H, NH2),2.32 (s, 3H, CH3). 13C-NMR(75.4 MHz, DMSO-d6): / ppm = 162.0, 160.2, 150.6, 133.9, 128.6, 124.1, 122.6, 110.0,20.7. IR: ν = 3426 (s), 3344 (w), 3066 (s), 2796 (w), 2681 (w), 1923 (w), 1734 (w), 1672 (s), 1626 (s), 1576 (s), 1516 (s), 1474 (m), 1455 (m), 1427 (s), 1399 (s), 1329 (m), 1289 (m), 1220 (w), 1172 (m), 1139 (w), 1092 (s), 1038 (m), 1002 (m), 962 (w), 922 (m), 874 (m), 857 (m), 824 (s),797 (m), 720 (m), 684 (m), 654 (m), 590 (m), 570 (s). Anal. calcd.for C9H10N4(174.20): C 62.05; N 5.79; 32.16; found: C 61.77; N 5.89; 32.40.
References:
Zeiger, Mirco;Stark, Sebastian;Kalden, Elisabeth;Ackermann, Bettina;Ferner, Jan;Scheffer, Ute;Shoja-Bazargani, Fatemeh;Erdel, Veysel;Schwalbe, Harald;Göbel, Michael W. [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 24,p. 5576 - 5580] Location in patent:supporting information
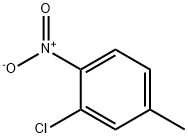
38939-88-7
197 suppliers
$6.00/1g

1955-61-9
8 suppliers
inquiry
608-05-9
254 suppliers
$10.00/5g

1955-61-9
8 suppliers
inquiry
64113-86-6
66 suppliers
inquiry

1955-61-9
8 suppliers
inquiry