
6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl synthesis
- Product Name:6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl
- CAS Number:753424-31-6
- Molecular formula:C14H17NO2
- Molecular Weight:231.29
![Spiro[2H-1-benzopyran-2,4'-piperidine]-1'-carboxylic acid, 3,4-dihydro-6-Methyl-4-oxo-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/958575-85-4.gif)
958575-85-4
7 suppliers
inquiry
![6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl](/CAS/20150408/GIF/753424-31-6.gif)
753424-31-6
16 suppliers
$167.00/250mg
Yield:753424-31-6 96%
Reaction Conditions:
Stage #1: tert-butyl 6-methyl-4-oxospiro[chroman-2,4′-piperidine]-1′-carboxylatewith trifluoroacetic acid in dichloromethane;
Stage #2: with sodium hydroxide in chloroform;water; pH=~ 12;
Steps:
21
A solution of 1-(2-hydroxy-5-methylphenyl)ethanone (100 g, 0.67 mol), tert-butyl 4-oxopiperidine-1- carboxylate (133 g, 0.67 mol) and pyrrolidine (55.8 mL, 0.67 mol) in methanol (600 mL) was stirred for 24 hours and filtered. The separated crystals were washed with hexane/ethyl acetate (9:1) mixture, then refluxed with hexane (150 mL) and separated by filtration again. The crystals were finally dried to give tert-butyl e-methyl^-oxo-S^-dihydro-i'H-spirotchromene^^'-piperidineJ-i'-carboxylate in 89% (195.5 g) yield. 1H NMR (DMSO-d6) δ 7.48 (s, 1H), 7.36 (dd, J=3, 8, 1 H), 6.94 (d, J=8, 1H)1 3.67 (m, 2H), 3.08 (m, 2H), 2.76 (s, 2H), 2.23 (s, 3H), 1.82 (m, 2H), 1.56 (m, 2H), 1.36 (s, 9H).Neat trifluoroacetic acid (80 mL) was added to a solution of tert-butyl 6-methyl-4-oxo-3,4-dihydro-1'H- spiro[chromene-2,4'-piperidine]-1'-carboxylate (40 g, 0.12 mol) in dichloromethane (300 mL). The obtained solution was stirred overnight and then evaporated. Water (200 mL) and chloroform (200 mL) were then added to the residue, and the obtained solution was made alkaline with 19 N NaOH to about pH 12. The product was then extracted with chloroform. The extract was passed through a layer of SiO2 and Na2SO4 and evaporated to give 6-methylspiro[chromene-2,4 -piperidin]-4(3H)-one in 96% (26.7 g) yield, 232 (ES+).
References:
WO2008/65508,2008,A1 Location in patent:Page/Page column 21-22
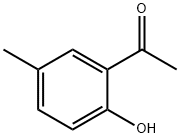
1450-72-2
196 suppliers
$6.00/1g
![6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl](/CAS/20150408/GIF/753424-31-6.gif)
753424-31-6
16 suppliers
$167.00/250mg

79099-07-3
543 suppliers
$5.00/5g
![6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl](/CAS/20150408/GIF/753424-31-6.gif)
753424-31-6
16 suppliers
$167.00/250mg

1450-72-2
196 suppliers
$6.00/1g

79099-07-3
543 suppliers
$5.00/5g
![6-Methylspiro[chroMan-2,4'-piperidin]-4-one hcl](/CAS/20150408/GIF/753424-31-6.gif)
753424-31-6
16 suppliers
$167.00/250mg