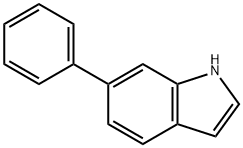
6-Phenyl-1H-indole synthesis
- Product Name:6-Phenyl-1H-indole
- CAS Number:106851-31-4
- Molecular formula:C14H11N
- Molecular Weight:193.24
Yield:106851-31-4 99%
Reaction Conditions:
with Ti0.97Pd0.03O1.97;potassium carbonate in lithium hydroxide monohydrate at 100; for 6 h;Suzuki-Miyaura Coupling;
Steps:
9.3 Typical experimental procedure for the palladium-catalyzed suzuki cross-coupling reaction in water
General procedure: A mixture of aryl halide 1 (0.4mmol), arylboronic acid 2 (0.8mmol), Ti0.97Pd0.03O1.97 (nanoparticles; 10wt %), K2CO3 (110mg, 0.8mmol) in water (2mL) was stirred at 100°C for 6h. After the completion of the reaction, diethyl ether (15ml x 3 times) was poured into the mixture, washed with water (15mL), extracted with diethyl ether (3×20mL), dried over anhydrous Na2SO4 and evaporated under vacuum; the residue was purified by column chromatography (petroleum ether or petroleum ether/ethyl acetate) to obtain the desired coupled product.
References:
Bhat K, Shrikanth;Lanke, Veeranjaneyulu;Prasad, Jagadeesh Dasappa;Prabhu, Kandikere Ramaiah [Applied Catalysis A: General,2020,vol. 596,art. no. 117516]
![[1,1'-Biphenyl]-3-amine, 4-(2,2-dimethoxyethyl)-](/CAS/20210305/GIF/106851-29-0.gif)
106851-29-0
0 suppliers
inquiry

106851-31-4
10 suppliers
inquiry
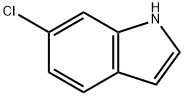
52415-29-9
414 suppliers
$9.00/1g

24388-23-6
208 suppliers
$6.00/5g

106851-31-4
10 suppliers
inquiry

52415-29-9
414 suppliers
$9.00/1g

106851-31-4
10 suppliers
inquiry