
6-phenylnaphtho[2,1-b]benzofuran synthesis
- Product Name:6-phenylnaphtho[2,1-b]benzofuran
- CAS Number:1383607-08-6
- Molecular formula:C22H14O
- Molecular Weight:294.35
Yield:1383607-08-6 56%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in ethanol;water;toluene at 80; for 2 h;Inert atmosphere;Suzuki-Miyaura Coupling;
Steps:
1 Synthesis of 6-phenylbenzo[b]naphtho[1,2-d]furan (abbreviation: PBnf)
Into a 200 mL three-neck flask were placed 0.90 g (5.7 mmol) of bromobenzene and 1.5 g (5.7 mol) of benzo[b]naphtho[1,2-d]furan-6-boronic acid, and the air in the flask was replaced with nitrogen. To this mixture were added 20 mL of toluene, 10 mL of ethanol, and 6.0 mL of an aqueous solution of sodium carbonate (2.0 mol/L). While the pressure was reduced, this mixture was stirred to be degassed. To this mixture was added 0.33 g (0.28 mmol) of tetrakis(triphenylphosphine)palladium(0), and the mixture was stirred at 80° C. for 2 hours under a nitrogen stream. After that, the aqueous layer of this mixture was subjected to extraction with toluene, and the obtained solution of the extract and the organic layer were combined and washed with saturated brine. The organic layer was dried with magnesium sulfate, and this mixture was gravity-filtered. An oily substance obtained by concentration of the obtained filtrate was purified by silica gel column chromatography (a developing solvent in which the ratio of hexane to toluene was 19:1) to give a white solid. The obtained solid was recrystallized with a mixed solvent of toluene and hexane, so that 0.95 g of white needle-like crystals of the object of the synthesis were obtained in 56% yield.
References:
US2012/165550,2012,A1 Location in patent:Page/Page column 19
![BENZO[B]NAPHTHO[1,2-D]FURAN](/CAS/GIF/205-39-0.gif)
205-39-0
77 suppliers
$127.11/1G
![6-phenylnaphtho[2,1-b]benzofuran](/CAS/20180527/GIF/1383607-08-6.gif)
1383607-08-6
15 suppliers
inquiry
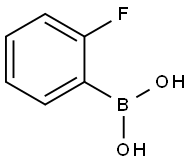
1993-03-9
350 suppliers
$8.00/1g
![6-phenylnaphtho[2,1-b]benzofuran](/CAS/20180527/GIF/1383607-08-6.gif)
1383607-08-6
15 suppliers
inquiry
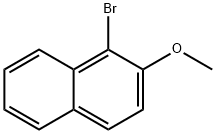
3401-47-6
165 suppliers
$59.00/5G
![6-phenylnaphtho[2,1-b]benzofuran](/CAS/20180527/GIF/1383607-08-6.gif)
1383607-08-6
15 suppliers
inquiry
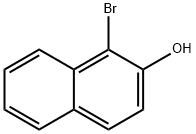
573-97-7
239 suppliers
$5.00/1g
![6-phenylnaphtho[2,1-b]benzofuran](/CAS/20180527/GIF/1383607-08-6.gif)
1383607-08-6
15 suppliers
inquiry

![Boronic acid, B-benzo[b]naphtho[1,2-d]furan-6-yl-](/CAS/20210305/GIF/1382763-54-3.gif)