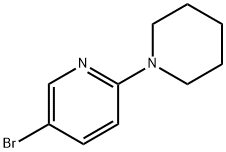
6-piperidin-1-ylnicotinonitrile synthesis
- Product Name:6-piperidin-1-ylnicotinonitrile
- CAS Number:501378-38-7
- Molecular formula:C11H13N3
- Molecular Weight:187.24

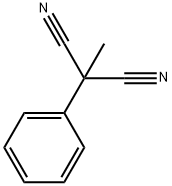
110-89-4
3 suppliers
$15.96/100ML
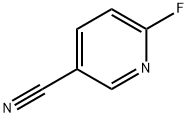
3939-12-6
132 suppliers
$6.00/250mg

501378-38-7
20 suppliers
inquiry
Yield:501378-38-7 89%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 100;
Steps:
General Procedure A: SNAR
To a round bottom flask containing DMF was added 6-fluoronicotinonitrile (1 equiv), piperidine (1 equiv), and the mixture was cooled to 0 °C in a brine ice bath. Sodium hydride (1.5 equiv) was then added and the reaction was allowed to warm to roomtemperature, followed by heating to 100 °C until consumption of starting maerial was observed as monitored by TLC (2-4 hours). Upon cooling to room temperature, the mixture was diluted in ethyl acetate and extracted with brine solution. The organic layers were then collected and dried over anhydrous sodium sulfate. Concentration in vacuo afforded the crude product as an oil, which was then purified by column chromatography (10-35% ethyl acetate in hexanes).
References:
WO2020/154431,2020,A1 Location in patent:Paragraph 00289-00291; 00302-00303

110-89-4
3 suppliers
$15.96/100ML
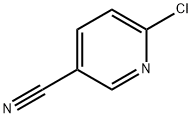
33252-28-7
431 suppliers
$6.00/1g

501378-38-7
20 suppliers
inquiry