
6-[(PYRIDINE-3-CARBONYL)-AMINO]-HEXANOIC ACID synthesis
- Product Name:6-[(PYRIDINE-3-CARBONYL)-AMINO]-HEXANOIC ACID
- CAS Number:110576-09-5
- Molecular formula:C12H16N2O3
- Molecular Weight:236.27
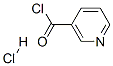
20260-53-1
201 suppliers
$16.00/5g
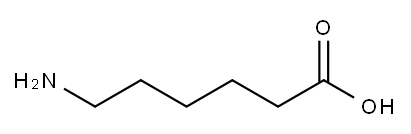
60-32-2
589 suppliers
$5.00/10g
![6-[(PYRIDINE-3-CARBONYL)-AMINO]-HEXANOIC ACID](/StructureFile/ChemBookStructure3/GIF/CB4478061.gif)
110576-09-5
9 suppliers
$378.00/1g
Yield:-
Reaction Conditions:
Stage #1: 6-aminohexanoic acidwith chloro-trimethyl-silane;triethylamine in dichloromethane at 0; for 2.75 h;Heating / reflux;
Stage #2: pyridine-3-carbonyl chloride hydrochloride in dichloromethane at 0 - 20;
Steps:
Synthesis of . 6-[(pyridin-3-carbonyl)-amino"l-esanoic acid (I) and 6- [(fenilcarbonyl)-amino]-esanoic acid (II)6-aminocaproic acid (l.Ommol) was suspended in dichloromethane, triethylamine (3.5mmol) and trimethylchlorosilane (l.lmmol) were then added and the suspension was refluxed under stirring for 2 hours.The obtained suspension was then cooled for 45 min at 0°C and thereafter 1 mmol of benzoyl chloride or nicotinoyl chloride hydrochloride was added. The suspension was stirred 24 hours at room temperature and then the solvent removed. The bulky residue was washed with cold water and the white solid obtained separated by centrifugation and dried under reduced pressure.
References:
WO2008/90585,2008,A2 Location in patent:Page/Page column 19; 20
78348-28-4
34 suppliers
$15.00/250mg

60-32-2
589 suppliers
$5.00/10g
![6-[(PYRIDINE-3-CARBONYL)-AMINO]-HEXANOIC ACID](/StructureFile/ChemBookStructure3/GIF/CB4478061.gif)
110576-09-5
9 suppliers
$378.00/1g