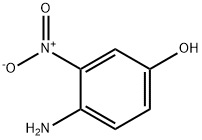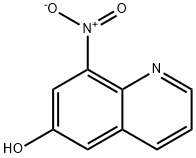
6-Quinolinol,8-nitro- synthesis
- Product Name:6-Quinolinol,8-nitro-
- CAS Number:5437-99-0
- Molecular formula:C9H6N2O3
- Molecular Weight:190.16
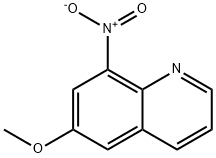
85-81-4
141 suppliers
$30.00/5g

5437-99-0
28 suppliers
inquiry
Yield:5437-99-0 95%
Reaction Conditions:
Stage #1: 6-methoxy-8-nitroquinolinewith lithium hydroxide monohydrate;hydrogen bromide for 16 h;Heating / reflux;
Stage #2: with sodium hydroxide;lithium hydroxide monohydrate
Steps:
35.1
Example 35; N-(6-(benzyloxy)quinolin-8-yl)-6-(sulfamoylamino)hexanamide (43) Step 1: 8-nitroquinolin-6-ol (38) 6-methoxy-8-nitroquinoline (2.15 g, 10.51 mmol) was dissolved in 48% HBr (8.0 mL, 147.20 mmol) and refluxed for 16 hours. The resulting suspension of yellow crystals was cooled down and then filtered. The solid was diluted in water and 15% NaOH (aq) was added. The suspension was filtered and the aqueous layer was acidified to obtain crystals at pH6. Crystals were washed with water and filtered to afford compound 38 as yellow crystals (1.89 g, 95%). LRMS (ESI): (calc.) 190.04 (found) 191.1 (MH)+.
References:
US2007/293530,2007,A1 Location in patent:Page/Page column 84
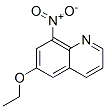
282547-59-5
5 suppliers
inquiry

5437-99-0
28 suppliers
inquiry
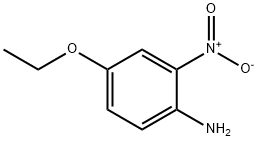
616-86-4
82 suppliers
$33.00/1g

5437-99-0
28 suppliers
inquiry
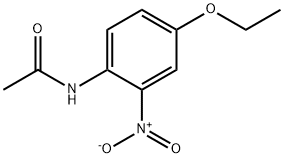
885-81-4
9 suppliers
inquiry

5437-99-0
28 suppliers
inquiry