
6-(tetrahydropyran-4-yloxy)nicotinonitrile synthesis
- Product Name:6-(tetrahydropyran-4-yloxy)nicotinonitrile
- CAS Number:884507-60-2
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
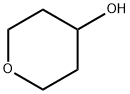
2081-44-9
288 suppliers
$6.00/1g
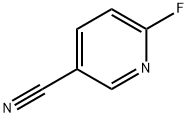
3939-12-6
132 suppliers
$6.00/250mg

884507-60-2
16 suppliers
inquiry
Yield:884507-60-2 78.9%
Reaction Conditions:
Stage #1: Tetrahydro-pyran-4-olwith sodium hydride in N,N-dimethyl-formamide; for 0.166667 h;Inert atmosphere;Cooling with ice;
Stage #2: 6-fluoropyridine-3-carbonitrile in N,N-dimethyl-formamide at 20; for 16 h;Inert atmosphere;Cooling with ice;
Steps:
46.1 Step 1: Synthesis of 6-((tetrahydro-2H-pyran-4-yl)oxy)-3-cyanopyridine
Under argon atmosphere, under ice-water bath, add sodium hydride (426 mg, 10.6 mmol) to sodium hydride (426 mg, 10.6 mmol)To the dry DMF (10.0 mL) solution was added tetrahydro-2H-pyran-4-ol (1.00 g, 9.83 mmol), and the reaction was stirred under an ice bath for 10 minutes before adding 6-fluoro-3-cyanopyridine ( 1.00g, 8.19 mmol) in dry DMF (5.00 mL).The reaction mixture was stirred at room temperature for 16 hours.To the reaction solution was added saturated aqueous ammonium chloride solution (30.0 mL). Extracted with EA (100 mL x 2). The combined organic phases were washed with saturated brine (25.0 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure to obtain the crude product. After separation and purification by column chromatography (PE:EA=10:1), the target compound (1.32 g, yield 78.9%, white solid) was obtained.
References:
CN114181205,2022,A Location in patent:Paragraph 0611-0614