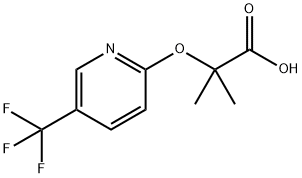
2-Methyl-2-[(5-trifluoromethylpyridin-2-yl)oxy]propionic acid synthesis
- Product Name:2-Methyl-2-[(5-trifluoromethylpyridin-2-yl)oxy]propionic acid
- CAS Number:605680-62-4
- Molecular formula:C10H10F3NO3
- Molecular Weight:249.19
Yield:-
Reaction Conditions:
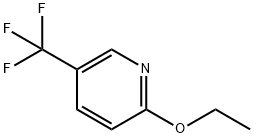
Stage #1: ethyl 2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanoatewith sodium hydroxide;water in tetrahydrofuran; for 22 h;Heating / reflux;
Stage #2: with hydrogenchloride in water; for 0.166667 h;
Steps:
14
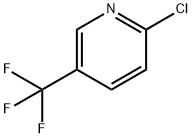
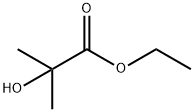
REFERENCE EXAMPLE 14; 2-Methyl-2-(5-trifluoromethyl-2-pyridyloxy)propionic Acid; Two nitrogen flushed, 12 L 3- necked round bottom flasks, each fitted with a thermometer and a reflux condenser were charged with KHMDS in THF (0.91 M, 3.52 L each, 3.205 mol, 1.5 eq). The solutions were cooled to - 700C and stirred magnetically. Ethyl-2-hydroxyisobutyrate (98%) (463 mL, 447g, 3.38 mol) was added to each flask over 30 min, keeping the reaction temperature below ~62°C. After 10 min 2- chloro-5-trifluo?nethylpyridine (388 g, 2.14 mol) was added to each flask in one portion. The cooling bath was removed and the reactions were allowed to warm to 200C overnight (ca 16 hr.).The reactions were monitored by TLC (silica, 90/10 Hex/EtOAc) and HPLC:
References:
WO2007/136571,2007,A1 Location in patent:Page/Page column 44-45
![Ethyl 2-methyl-2-[[5-(trifluoromethyl)pyridin-2-yl]oxy]propanoate](/CAS/GIF/913849-17-9.gif)
913849-17-9
15 suppliers
inquiry
![2-Methyl-2-[(5-trifluoromethylpyridin-2-yl)oxy]propionic acid](/CAS/GIF/605680-62-4.gif)
605680-62-4
32 suppliers
inquiry
33252-63-0
288 suppliers
$10.00/1g
![2-Methyl-2-[(5-trifluoromethylpyridin-2-yl)oxy]propionic acid](/CAS/GIF/605680-62-4.gif)
605680-62-4
32 suppliers
inquiry