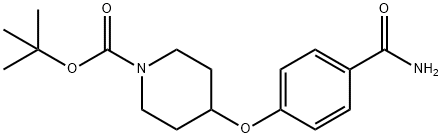
tert-Butyl 4-(4-carbaMoylphenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-carbaMoylphenoxy)piperidine-1-carboxylate
- CAS Number:609781-33-1
- Molecular formula:C17H24N2O4
- Molecular Weight:320.38
619-57-8
205 suppliers
$5.00/1g

109384-19-2
469 suppliers
$5.00/1g

609781-33-1
30 suppliers
$85.00/100mg
Yield:609781-33-1 21%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran at 0 - 20;
Steps:
24.a Example 24; Synthesis of 4-(piperidin-4-yloxy)benzamide trifluoroacetate; (a) Synthesis of tert-butyl 4-[4-(aminocarbonyl)-phenoxy]piperidine-1-carboxylate
(a) Synthesis of tert-butyl 4-[4-(aminocarbonyl)-phenoxy]piperidine-1-carboxylate To a solution of p-hydroxybenzamide (343 mg, 2.50 mmol) in tetrahydrofuran (10 ml) were added tert-butyl 4-hydroxy-1-piperidinecarboxylate (503 mg, 2.50 mmol) and triphenylphosphine (656 mg, 2.50 mmol), followed by adding thereto ethyl azodicarboxylate (1.15 ml, 2.50 mmol) under ice-cooling, and the resulting mixture was stirred overnight at room temperature. Then, the reaction mixture was concentrated and the crude product thus obtained was purified by a silica gel column chromatography (chloroform/methanol = 99/1) to obtain tert-butyl 4-[4-(aminocarbonyl)phenoxy]-piperidine-1-carboxylate (169 mg, 21%). 1H-NMR (DMSO-d6) δ; 1.39(s, 9H), 1.45-1.6 0(1H, m), 1.89-1.92 (1H, m), 3.13-3.20(2H, m), 3.62 - 3.69 (2H, m), 4.61-4.66 (1H, m), 6. 99 (2H, d, J=8.8Hz), 7.15(1H, m), 7.80 (1H, m), 7.81 (2H, d, J=8.8Hz).
References:
EP1500643,2005,A1 Location in patent:Page 24
333954-86-2
37 suppliers
inquiry

609781-33-1
30 suppliers
$85.00/100mg