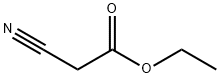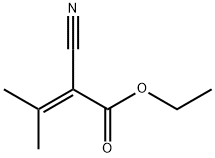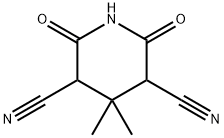
4,4-Dimethyl-2,6-dioxopiperidine-3,5-dicarbonitrile synthesis
- Product Name:4,4-Dimethyl-2,6-dioxopiperidine-3,5-dicarbonitrile
- CAS Number:61193-04-2
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19
Yield:61193-04-2 51%
Reaction Conditions:
with ammonium acetate;ammonia at 5;
Steps:
3-(R,S),5-(R,S)-3,5-Dicyano-4,4-dimethyl-2,6-dioxopiperidine (29)
Compound 29 was prepared according to the previously described method [44]. Ethyl cyanoacetate (169.7 g; 1.5 mol), acetone (43.5 g; 0.75 mol), ammonium acetate (1.4 g) and 300mL of ethanol, which contained 30 g of ammonia, were placed into 1-l flask. The mixture was kept overnight at 5 °C. The precipitated ammonium salt was filtered off using a Büchner funnel and then was washed with 100 mL of diethyl ether. The precipitate was dissolved in 350 mL of water (80 °C), and the aqueous solution was acidified (pH ~ 1) by adding concentrated hydrochloric acid. The flask was cooled on an ice bath, and the crystals appeared almost immediately. The product was filtered off using a Büchner funnel, washed with 250 mL of water and dried under high vacuum. Yield: 73 g (51%). White solid, m.p. 218-220 °C (lit. [45] 215-217 °C), Rf = 0.40 (S4). 1H NMR (600 MHz, DMSO-d6): δ = 4.71 (s, 2H), 1.35 (s, 3H, CH3), 1.18 (s, 3H, CH3). 13C NMR (150.9 MHz, DMSO-d6): δ = 164.71 (2 CO-O), 114.53 (2 x CN), 47.03 (2C), 36.41, 26.08, 19.86. IR (KBr) nmax (cm -1): 2272 w, 2260 w (CN); 3219 s, 3129 m, 810 m, 791 m, 747 m (NH); 1744 vs, 1726 vs, 1714 vs (C=O); 1244 s, 1230 s (amide II); 2975 m, 1367 s (CH3). HRMS (ESI) calc for C9H9O2N3Na [M + Na]+ 214.05909, found: 214.05901.
References:
Pi?ha, Jan;Vaňek, Václav;Budě??sińsky, Milo?;Mlad?ková, Jana;Garrow, Timothy A.;Ji??acek, Ji??i [European Journal of Medicinal Chemistry,2013,vol. 65,p. 256 - 275]

67-64-1
6 suppliers
$19.10/10ml
107-91-5
538 suppliers
$5.00/10g

61193-04-2
11 suppliers
inquiry
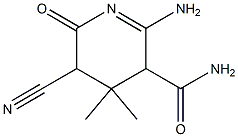
6630-38-2
0 suppliers
inquiry

110-89-4
3 suppliers
$15.96/100ML

67-64-1
6 suppliers
$19.10/10ml

107-91-5
538 suppliers
$5.00/10g

61193-04-2
11 suppliers
inquiry

6630-38-2
0 suppliers
inquiry