
2-(2-CHLORO-ACETYLAMINO)-4-METHYL-THIAZOLE-5-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-(2-CHLORO-ACETYLAMINO)-4-METHYL-THIAZOLE-5-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:6125-37-7
- Molecular formula:C9H11ClN2O3S
- Molecular Weight:262.71

7210-76-6
247 suppliers
$5.00/1g

79-04-9
352 suppliers
$12.00/5g

6125-37-7
20 suppliers
$200.00/500mg
Yield:6125-37-7 90%
Reaction Conditions:
Stage #1: ethyl-2-amino-4-methylthiazole-5-carboxylatewith potassium carbonate in dichloromethane at 20; for 0.5 h;
Stage #2: chloroacetyl chloride in dichloromethane at 20;
Steps:
2
50 mmol of 2-amino-4-methylthiazole-5-carboxylic acid ethyl ester, 100 mmol of anhydrous potassium carbonate, 100 mL of dichloromethane, stirred at room temperature for 30 min, dropping 8. OmL of chloroacetyl chloride at room temperature The The reaction solution was poured into ice water, extracted with dichloromethane, washed with saturated brine, washed with saturated sodium carbonate solution, washed with water, dried over anhydrous sodium sulfate, and the crystals were recrystallized from ethanol / water to obtain white crystals 2-(2-chloroacetylamino)-4-methylthiazole-5-carboxylic acid ethyl ester in 90%
References:
CN106632134,2017,A Location in patent:Paragraph 0027-0030

7210-76-6
247 suppliers
$5.00/1g

6125-37-7
20 suppliers
$200.00/500mg
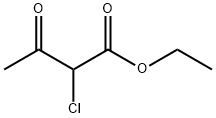
609-15-4
446 suppliers
$6.00/25g

6125-37-7
20 suppliers
$200.00/500mg
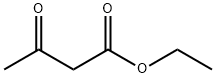
141-97-9
781 suppliers
$5.00/25g

6125-37-7
20 suppliers
$200.00/500mg