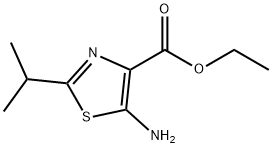
4-Thiazolecarboxylic acid, 5-amino-2-(1-methylethyl)-, ethyl ester synthesis
- Product Name:4-Thiazolecarboxylic acid, 5-amino-2-(1-methylethyl)-, ethyl ester
- CAS Number:612505-08-5
- Molecular formula:C9H14N2O2S
- Molecular Weight:214.28
![Acetic acid, 2-cyano-2-[(2-methyl-1-oxopropyl)amino]-, ethyl ester](/CAS/20210305/GIF/612505-07-4.gif)
612505-07-4
0 suppliers
inquiry

612505-08-5
3 suppliers
inquiry
Yield:612505-08-5 63%
Reaction Conditions:
with Lawessons reagent in toluene at 70; for 12 h;
Steps:
80
Stir cyano-isobutyrylamino-acetic acid ethyl ester (139 g, 701 mmol) mechanically stir as a slurry in toluene (1.4 L) at rt under N2. Add lawesson's reagent (170 g, 420 mmol, 0.6 eq. ) in portions and heat the thick slurry to [70 °C] and stir for 12 hours until complete by TLC (2: 1/heptane : THF). Cool the mixture and concentrate to dryness in vacuo on a rotovapor to obtain 353 g of thick yellow oil that was partially purified by silica gel plug (1 Kg silica gel 60,1. 5 vol. warm 2: 1/THF : heptane as diluent, 2: 1/heptane : THF as eluent). Combine the product containing filtrates and concentrate to dryness in vacuo on a rotovapor to obtain 194 g of crude solid. Dissolve the solid in EtOAc (400 mL) at [50-60 °C] with stirring, then allow to cool gradually to rt. Precipitate the product and was cool to [0-5 °C] with stirring for 30 minutes, isolate by suction filtration, rinse with cold EtOAc (2 x 50 mL), then dry in a vacuum oven at [50 °C] to afford a first crop of 76.3 g [(51 %)] of the title compound. Obtain a second crop of 17.6 g (12%) = from the filtrate after concentration in vacuo and silica gel chromatography (1 Kg silica gel 60,2 : 1/heptane : [THF).'H] NMR [(300] MHz, DMSO-d6) 8 7.23 (bs, 2H), 4.21 (q, 2H, [J= 6.] 95 Hz), 3.02 (dq, [1H,] [J= 6.] 95 Hz), 1.27 (t, 3H, [J= 6.] 95 Hz), 1.22 (d, 6H, J= 6.95 [HZ).'3C] NMR (75 MHz, DMSO-d6) [8] 163.72, 160.08, 156.20, 118.08, 58.99, 32.27, 22.35 [(2),] 14.46. IR [(CHC13)] 3483,3347, 2975,2933, 2868,1668, 1582,1530, 1494, 1464,1409, [1382CM-1.] HRMS (ES) M/z calculated for [C9HL4N202S] 215. [0854,] found 215.0842.
References:
WO2004/14895,2004,A1 Location in patent:Page 93
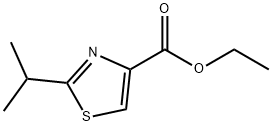
133047-44-6
56 suppliers
$20.00/100mg

612505-08-5
3 suppliers
inquiry
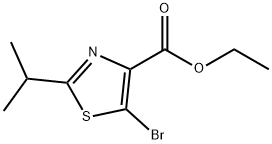
848555-51-1
1 suppliers
inquiry

612505-08-5
3 suppliers
inquiry

70-23-5
319 suppliers
$6.00/5g

612505-08-5
3 suppliers
inquiry