
Ethyl2-(4-Bromophenyl)-4-methylthiazole-5-carboxylate synthesis
- Product Name:Ethyl2-(4-Bromophenyl)-4-methylthiazole-5-carboxylate
- CAS Number:61291-89-2
- Molecular formula:C13H12BrNO2S
- Molecular Weight:326.21

26197-93-3
87 suppliers
$17.89/1g
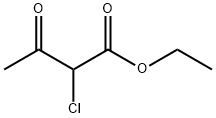
609-15-4
447 suppliers
$6.00/25g

61291-89-2
12 suppliers
$250.00/5g
Yield:61291-89-2 73%
Reaction Conditions:
in ethanol; for 5 h;Inert atmosphere;Reflux;
Steps:
2.3.2. General procedure for the synthesis of compounds3a-3e from compounds 2a-2e
General procedure: The synthetic procedure of compounds 3a-3e: To a stirredsolution of 2a-2e (20 mmol) in EtOH (40 mL) and was addedethyl 2-chloro-3-oxobutanoate (20 mmol, 3.29 g), the reactionmixture was heated at reflux in N2 atmosphere until the completion of reaction as indicated by TLC analysis (typicallywithin 5 h).The reaction mixture was poured into ice-water (100 mL)and the mixture thus obtained was extracted with CH2Cl2(100 mL 3). The combined organic phases were washedwith saturated brine (300 mL), dried over anhydrous Na2SO4and evaporated on a rotary evaporator to afford a residue,which was purified by column chromatography through ashort silica gel column to yield 3a-3e.
References:
Wu, Jingwei;Li, Weiya;Zheng, Zhihui;Lu, Xinhua;Zhang, Huan;Ma, Ying;Wang, Runling [Journal of Biomolecular Structure and Dynamics,2021,vol. 39,# 4,p. 1174 - 1188]
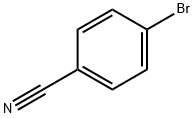
623-00-7
466 suppliers
$6.00/10g

61291-89-2
12 suppliers
$250.00/5g