5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole synthesis
- Product Name:5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole
- CAS Number:6148-18-1
- Molecular formula:C9H7ClN2
- Molecular Weight:178.62
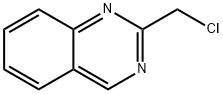
6141-22-6
7 suppliers
inquiry
![5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole](/CAS/GIF/6148-18-1.gif)
6148-18-1
57 suppliers
$67.00/100mg
Yield:6148-18-1 53.2%
Reaction Conditions:
with ammonia in methanol at 20; for 4.5 h;Sealed tube;
Steps:
2 Step 2:
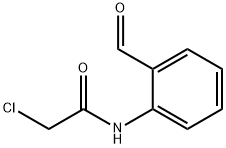
The product of the first step, 2-chloro-N-(2-formylphenyl)acetamide (0.869 g, 4.40 mmol) and ammonia, 7N solution in methanol (4.4 mL, 30.8 mmol) were stirred together at ambient in a sealed tube for 4.5 hours. A TLC (9:1 hexane EtOAc) check indicated that a new lower Rf spot had formed, however, plenty of starting material remained so stirring at ambient temperature was continued for another 19.5 hours, during which time a white precipitate had formed. Volatiles were removed under reduced pressure to give a beige solid that was treated with CHCl3. The portion that was soluble was checked by TLC (7:3 hexane/EtOAc). The major spot in the CHCl3-soluble portion was a new one with lower Rf than the starting material. The CHCl3 mixture was placed in the 0° C. freezer over the weekend. Insoluble material was removed by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on an Analogix IF-280 (Agilent SF10-4 g, 100% CHCl3). The product eluted in fractions 1-10. The product fractions were combined and concentrated under reduced pressure to give 2-(chloromethyl)quinazoline as a white solid (418 mg, 53.2%). 1H NMR (300 MHz, CDCL3) δ 9.45 (s, 1H), 8.09 8.04 (m, 1H), 7.99-7.92 (m, 2H), 7.70 (ddd, J=8.0, 6.9, 1.2 Hz, 1H), 4.92 (s, 2H).
References:
US2013/343992,2013,A1 Location in patent:Paragraph 0302
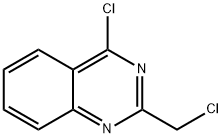
34637-41-7
35 suppliers
$180.00/1g
![5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole](/CAS/GIF/6148-18-1.gif)
6148-18-1
57 suppliers
$67.00/100mg
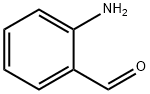
529-23-7
244 suppliers
$26.00/1g
![5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole](/CAS/GIF/6148-18-1.gif)
6148-18-1
57 suppliers
$67.00/100mg
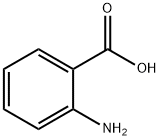
118-92-3
4 suppliers
$25.89/25g
![5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole](/CAS/GIF/6148-18-1.gif)
6148-18-1
57 suppliers
$67.00/100mg
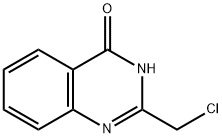
3817-05-8
54 suppliers
$60.00/50mg
![5-[(4-chlorophenoxy)methyl]-3-[(4-methoxyphenyl)methyl]-1,2,4-oxadiazole](/CAS/GIF/6148-18-1.gif)
6148-18-1
57 suppliers
$67.00/100mg