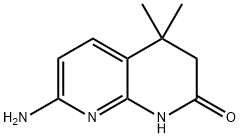
7-aMino-4,4-diMethyl-3,4-dihydro-1,8-naphthyridin-2(1H)-one synthesis
- Product Name:7-aMino-4,4-diMethyl-3,4-dihydro-1,8-naphthyridin-2(1H)-one
- CAS Number:618446-06-3
- Molecular formula:C10H13N3O
- Molecular Weight:191.23
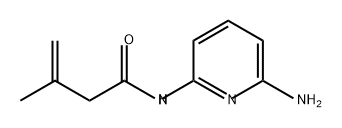
846035-54-9

618446-06-3
Example 4: 7-Amino-4,4-dimethyl-3,4-dihydro-1H-[1,8]naphthyridin-2-one was synthesized as follows: 1. 3-Methyl-but-3-enoic acid (6-amino-pyridin-2-yl)amide (49.2 g, 0.26 mol) was dissolved in 500 mL of dichloromethane (CH2Cl2) in a 1000 mL three-necked flask equipped with a mechanical stirrer, a 125 mL dosing funnel and a thermocouple. 2. Methanesulfonic acid (MeSO3H, 50 mL, 0.78 mol) was slowly added dropwise to the flask under stirring conditions. The temperature of the reaction was controlled by an ice/water bath to ensure that the temperature was below 20°C. After dropwise addition, continue stirring for 15 minutes. 3. Aluminum trichloride (AlCl3, 274 g, 2.08 mol) was suspended in 1500 mL of dichloromethane in another 5 L four-necked flask equipped with a mechanical stirrer, 1000 mL dosing funnel, N2 inlet, and thermocouple. 4. The amide solution prepared in step 2 was slowly added dropwise to the aluminum trichloride suspension. The temperature of the reaction was controlled by an ice/water bath, keeping the temperature below 20°C. After the dropwise addition was completed, the reaction mixture was allowed to gradually warm up to room temperature and stirred overnight. Upon completion of the reaction, complete consumption of the β,γ-unsaturated isomer was confirmed by TLC. 5. The reaction mixture was slowly poured into ice water for reverse quenching. The pH of the quenched mixture was adjusted to 8-10 with 2 N potassium hydroxide (KOH) solution.At this point, the salts precipitated out of solution and the aqueous phase was saturated. 6. The suspension was transferred to a partition funnel and extracted twice with a solvent mixture of dichloromethane/ethanol/ammonia (100:8:1, v/v/v). The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated to give the crude product. 7. The crude product was ground with ethyl acetate (EtOAc) and filtered to afford 7-amino-4,4-dimethyl-3,4-dihydro-1H-[1,8]naphthyridin-2-one (22.4 g, 0.117 mol, 46% yield). Mass spectrometry analysis (APCI): m/z 192.2 (M+1, calculated exact mass: 191.11).

846035-54-9
0 suppliers
inquiry

618446-06-3
24 suppliers
$57.00/100mg
Yield:618446-06-3 46%
Reaction Conditions:
Stage #1: 3-Methyl-but-3-enoic acid (6-amino-pyridin-2-yl)-amidewith methanesulfonic acid in dichloromethane at 20; for 0.25 h;
Stage #2: with aluminum (III) chloride in dichloromethane at 20;
Stage #3: with potassium hydroxide;water pH=8 - 10;
Steps:
4
EXAMPLE 47-Amino-4,4-dimethyl-3,4-dihydro-lH-[l,8]naphthyridin-2-one; 7-Amino-4,4-dimethyl-3,4-dihydro-lH-[l,8]naphthyridin-2-one, was produced as follows: 3-Methyl-but-3-enoic acid (6-amino-pyridin-2-yl)-amide (49.2 g, 0.26 mol) was dissolved in 500 mL CH2Cl2 in a 1000 mL 3-neck flask equipped with mechanical stirring, a 125 mL addition funnel and a thermal couple. While stirring, MeSO3H (50 mL, 0.78 mol) was added to the flask dropwise. The exotherm upon addition was controlled to maintain a temperature <20 0C by an ice/water bath. The mixture was allowed to stir for 15 minutes. AlCl3 (274 g, 2.08 mol) was suspended in 1500 mL CH2Cl2 in a 5 L 4-neck flask equipped with mechanical stirring, 1000 mL addition funnel, N2 line and a thermal couple. To this suspension, the amide solution was added dropwise. The exotherm from the addition was again controlled to maintain a temperature <200C with an ice/water bath. The reaction was allowed to warm to room temperature and stir overnight. The reaction had consumed all the beta gamma unsaturated isomer and was deemed complete. The reaction mixture was slowly added to ice as an inverse quench. The quenched mixture was brought to pH 8-10 with 2 N KOH. The salts precipitated out of solution and saturated the aqueous phase. The suspension was transferred to a separatory funnel and extracted twice with 100:8:1 CH2C^EtORNH4OH. The organic layers were combined, dried over Na2SO4, filtered and concentrated to a crude solid. The solid was triturated with EtOAc and filtered. The resulting solid was 7-Amino-4,4-dimethyl-3,4-dihydro-lH-[l,8]naphthyridin-2-one (22.4 g, 0.117 mol, 46%). MS: APCI: M+l: 192.2 (Exact Mass: 191.11). EPO
References:
WO2006/90272,2006,A1 Location in patent:Page/Page column 32
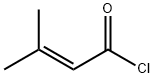
3350-78-5
127 suppliers
$31.80/1gm:

618446-06-3
24 suppliers
$57.00/100mg

141-86-6
514 suppliers
$6.00/5g

618446-06-3
24 suppliers
$57.00/100mg