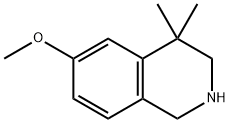
6-Methoxy-4,4-diMethyl-1,2,3,4-tetrahydroisoquinoline synthesis
- Product Name:6-Methoxy-4,4-diMethyl-1,2,3,4-tetrahydroisoquinoline
- CAS Number:62245-15-2
- Molecular formula:C12H17NO
- Molecular Weight:191.27
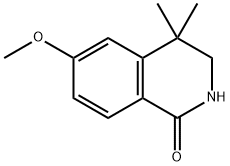
60812-60-4
1 suppliers
inquiry

62245-15-2
3 suppliers
inquiry
Yield:62245-15-2 100%
Reaction Conditions:
in tetrahydrofuran;chloroform;
Steps:
6-Methoxy-4,4-dimethyl-1,2,3,4-tetrahydro-isoquinoline (Intermediate 18)
6-Methoxy-4,4-dimethyl-1,2,3,4-tetrahydro-isoquinoline (Intermediate 18) A solution of 6-methoxy-4,4-dimethyl-1,2,3,4-tetrahydro-isoquinoline-1-one (Intermediate 17, 3.5 g, 17 mmol) in 100 mL of anhydrous tetrahydrofuran was treated with lithium aluminum hydride (1.3 g, 34.25 mmol) in small portions and the resulting suspension was refluxed for 3 hours under argon. The reaction mixture was then cooled in an ice bath and cautiously quenched with saturated aqueous sodium sulfate solution and the resulting slurry was filtered and the filter-cake washed well with ethyl acetate. The filtrate and washings were evaporated in vacuo to a brown oil which was dissolved in chloroform, the solution was dried over anhydrous magnesium sulfate, filtered and evaporated in vacuo to afford the title compound (3.2 g, ~100%). 1H-NMR (300 MHz, CDCl3): δ1.27 (s, 6H), 2.22 (s, 1H), 2.84 (s, 2H), 3.79 (s, 3H), 3.95 (s, 2H), 6.68(dd, J=2.4 Hz, 8.3 Hz, 1H), 6.86(d, J=2.4 Hz, 1H), 6.91 (d, J=8.3 Hz, 1H).
References:
US6313107,2001,B1
60812-46-6
16 suppliers
$65.00/25mg

62245-15-2
3 suppliers
inquiry
