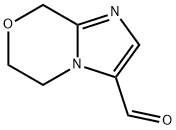
8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI) synthesis
- Product Name:8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)
- CAS Number:623564-43-2
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
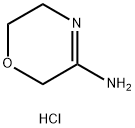
623564-41-0
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
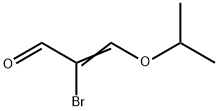
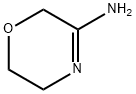
Step 5: Synthesis of 5,6-dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde (9) and 6,8-dihydro-5H-imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde: A mixture of 2-bromo-3-hydroxyacrylaldehyde (4.1 g), p-toluenesulfonic acid monohydrate (52 mg) and 2-propanol (5.2 mL) in cyclohexane ( The mixture in 42 mL) was subjected to azeotropic dehydration until the vapor temperature rose to 80 °C. The reaction mixture was concentrated under reduced pressure and the residue was dissolved in anhydrous ethanol (50 mL). Subsequently, a mixture of anhydrous ethanol (200 mL) solution of 3-iminomorpholine hydrochloride (3.4 g) and 28% methanol solution of sodium methanolate (4.8 g) was added to this solution at room temperature. The reaction mixture was stirred at room temperature for 2 hours before the solvent was removed under vacuum. The residue was dissolved in chloroform (125 mL), triethylamine (3.5 mL) was added, and then the reaction mixture was heated to reflux for 2 hours. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was dissolved in dichloromethane (300 mL) and washed with 50% aqueous K2CO3 (2 x 100 mL). The organic layer was dried over anhydrous MgSO4 and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography using CHCl3-acetone (4:1) as eluent to afford the target product 5,6-dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde (light orange solid, 1.4 g, 36.3% yield) and the by-products 6,8-dihydro-5H-imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde (light orange solid, 609 mg, 16.1% yield). 1H NMR (CDCl3) data of the target product: δ 4.08-4.15 (m, 4H), 4.88 (s, 2H), 7.58 (s, 1H), 9.85 (s, 1H). 1H NMR (CDCl3) data of the by-product: δ 4.06 (t, 2H, J = 5.2Hz), 4.40 (t, 2H, J = 5.2Hz), 4.90 (s, 2H), 7.75 (s, 1H), 9.72 (s, 1H).

623564-41-0
4 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
40 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
27 suppliers
inquiry
Yield:623564-42-1 36.3% ,623564-43-2 16.1%
Reaction Conditions:
Stage #1: 2-bromo-3-hydroxypropenalwith toluene-4-sulfonic acid;isopropyl alcohol in cyclohexane at 80;
Stage #2: morpholin-3-ylideneamine hydrochloridewith sodium methylate in methanol;ethanol at 20; for 2 h;
Stage #3: with triethylamine in chloroform; for 2 h;Heating / reflux;
Steps:
7.5
Step 5: 5,6-Dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carbaldehyde (9) and 56- dihvdro-8H-imidazof2, 1-clf1, 410xazine-3-carbaldehYde; The mixture of 2-bromo-3-hydroxypropenal (4.1 g), p-toluenesulfonic acid monohydrate (52 mg) and 2-propanol (5.2 mL) in cyclohexane (42 mL) was azeotroped until the vapor temperature rose to 80°C. The reaction mixture was concentrated under reduce pressure. The residue was dissolved in dry ethanol (50 mL). A mixture of the dry ethanol (200 mL) solution of 3-iminomorpholin hydrochloride (3.4 g) and 28% methanol solution of sodium methylat (4.8 g) was added at room temperature. The reaction mixture was stirred at room temperature for 2 h, and then the reaction solvent was removed in vacuo. The residue was dissolved in chloroform (125 mL) and triethylamine (3.5 mL) was added, then the reaction mixture was heated to reflux for 2 h. The reaction mixture was cooled to room temperature and then concentrated under reduce pressure. The residue was dissolved in dichloromethane (300 mL) and washed with 50% K2CO3 aqueous solution (2 x 100 mL). The organic layer was dried (MgS04) and filtered. The filtrate was concentrated under reduce pressure. The residue was applied to silica gel column chromatography and eluted with CHCI3-acetone (4: 1) to obtain the title (pale orange solid, 1.4 g, 36.3%) and the other regio isomer. (pale orange solid, 609 mg, 16. 1%). Desired product :'H NMR (CDCI3) 8 4.08-4. 15 (m, 4H), 4.88 (s, 2H), 7.58 (s, 1H), 9.85 (s, 1H). The unwanted regio isomer :'H NMR (CDCI3) 8 4.06 (t, 2H, J = 5.2 Hz), 4.40 (t, 2H, J = 5. 2 Hz), 4.90 (s, 2H), 7.75 (s, 1H), 9.72 (s, 1H).
References:
WO2003/93279,2003,A1 Location in patent:Page/Page column 65-66

623564-41-0
4 suppliers
inquiry

19263-02-6
1 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
40 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
27 suppliers
inquiry

623564-41-0
4 suppliers
inquiry
155272-73-4
7 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
40 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
27 suppliers
inquiry
747408-16-8
9 suppliers
inquiry

155272-73-4
7 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
40 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
27 suppliers
inquiry