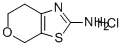
6,7-DIHYDRO-4H-PYRANO[4,3-D]THIAZOL-2-YLAMINE HYDROCHLORIDE synthesis
- Product Name:6,7-DIHYDRO-4H-PYRANO[4,3-D]THIAZOL-2-YLAMINE HYDROCHLORIDE
- CAS Number:623931-31-7
- Molecular formula:C6H9ClN2OS
- Molecular Weight:192.67
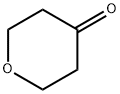
29943-42-8
431 suppliers
$5.00/1g
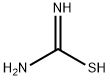
17356-08-0
3 suppliers
inquiry
![6,7-DIHYDRO-4H-PYRANO[4,3-D]THIAZOL-2-YLAMINE HYDROCHLORIDE](/CAS/GIF/623931-31-7.gif)
623931-31-7
26 suppliers
$189.00/250mg
Yield:623931-31-7 47%
Reaction Conditions:
Stage #1: Tetrahydro-4H-pyran-4-onewith sulfuryl dichloride in tetrachloromethane at 0 - 20; for 4 h;
Stage #2: with water at 0;
Stage #3: thiourea in tetrahydrofuran;ethanol; for 8 h;Heating / reflux;
Steps:
15.1
A mixture of tetrahydro-4H-pyran-4-one (5.0 g, 50 mmol) in CCl4 (100 ml) at 0° C., SO2Cl2 (7.4 g, 55 mmol) was slowly added. After the addition, reaction mixture was stirred at room temperature for 4 hrs and carefully quenched with ice cold water. Recation mixture was washed well and dried over anhydrous MgSO4. The organic layer was filtered and concentrated. The colurless oil obtained was diisoolved in THF/EtOH containing thiourea (4.0 g, 52 mmol) and refluxed for 8 hrs. At the end, reaction mixture was cooled to room temperature and the separated, 6,7-dihydro-4H-pyrano[4,3-d][1,3]thiazol-2-amine hydrochloride white solid was filtered. Yield. 4.5 g (47%); M.Pt. 115° C., (M+H) 157. To a stirred mixture of, 6,7-dihydro-4H-pyrano[4,3-d][1,3]thiazol-2-amine hydrochloride (4.0 g, 20.8 mmol) was dissolved in anhydrous ethanol (100 ml) and sodium methoxide (1.1 g, 21 mmol). This was stirred at room temperature for 30 minutes and to this ethyl bromopyruvate (10.0 g) was added and stirred at room temperature for 2 hrs. Then it was refluxed for 48 hrs. At the end reaction mixture was cooled to room temperature and concentrated. The residue was extracted with chloroform and washed well with water. The product was purified by silica-gel column chromatography by eluding it with 50% ethyl acetae:hexane. Red semi-solid; Yield: 3.1 g, (59%) M+H 253. The ester was reduced with LiBH4 and the resultant alcohol was oxidized with active MnO2. The aldehyde obtained was taken to next step.
References:
US2006/276446,2006,A1 Location in patent:Page/Page column 19

17356-08-0
3 suppliers
inquiry
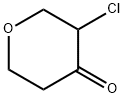
160427-98-5
28 suppliers
$117.00/50mg
![6,7-DIHYDRO-4H-PYRANO[4,3-D]THIAZOL-2-YLAMINE HYDROCHLORIDE](/CAS/GIF/623931-31-7.gif)
623931-31-7
26 suppliers
$189.00/250mg
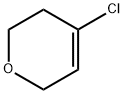
24265-21-2
4 suppliers
inquiry

17356-08-0
3 suppliers
inquiry
![6,7-DIHYDRO-4H-PYRANO[4,3-D]THIAZOL-2-YLAMINE HYDROCHLORIDE](/CAS/GIF/623931-31-7.gif)
623931-31-7
26 suppliers
$189.00/250mg