
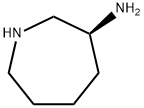
(3S)-3-AMinoazepane-1-carboxylic Acid tert-Butyl Ester synthesis
- Product Name:(3S)-3-AMinoazepane-1-carboxylic Acid tert-Butyl Ester
- CAS Number:625471-04-7
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3

13139-12-3
176 suppliers
$5.00/1g
107885-67-6
50 suppliers
inquiry

625471-04-7
83 suppliers
$35.00/100mg
Yield:625471-04-7 54%
Reaction Conditions:
in dichloromethane at -78 - 20; for 4.16667 - 4.25 h;
Steps:
26
Example 26 2-R (AMINOCARBONYL) AMINOL-N-R (3S)-AZEPAN-3-VLL-5-(4-METHOXD7PHENVL) THIOPHENE-3 carboxamide hydrochloride (S)-3-AMINO-AZEPANE-1-CARBOXYLIC acid tert-butyl ester. (S)-Azepan-3-ylamine (5g ; 43.8 mmol) was dissolved in 100 mL of anhydrous CH2C12 and cooled to-78 °C while stirring with a magnetic stirring bar. In another flask N-(TERT-BUTOXYCARBONYLOXY) SUCCINIMIDE [BOC-OSU] (9.7 g; 45 mmol) was dissolved in 50 ML of anhydrous CH2C12. To the stirred solution of the amine was added the solution of the SUCCINIMIDE over a period of 10-15 minutes so as to keep the reaction mixture at-78 °C while stirring. After the addition was complete, the reaction was allowed to warm to room temperature and then stirred for an additional 4h or until the reaction was complete by TLC (Ninhydrin; Rf 0.3 ; 0.1 : 1: 10 NH4OH, MeOH ; CH2C12). The reaction mixture was washed with 50 mL of H20. The aqueous layer was brought to a pH >13 by the addition of 6N NAOH and extracted with CH2C12 (3 x 100 mL). The organic layer was dried over Na2C03, filtered, and concentracted in a vacuum to yield pure (S)-3-AMINO-AZEPANE-L-CARBOXYLIC acid tert- butyl ester as a viscous oil (5.1 g, 54%).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2005/16909, 2005, A1 Location in patent:Page/Page column 68
870842-23-2
120 suppliers
$80.00/250mg

625471-04-7
83 suppliers
$35.00/100mg