
[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-(5-METHYL-FURAN-2-YLMETHYL)-AMINE synthesis
- Product Name:[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-(5-METHYL-FURAN-2-YLMETHYL)-AMINE
- CAS Number:626216-00-0
- Molecular formula:C16H21NO3
- Molecular Weight:275.34
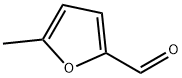
620-02-0
477 suppliers
$14.14/5gm:
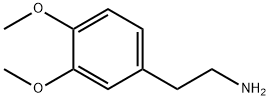
120-20-7
543 suppliers
$5.00/5G
![[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-(5-METHYL-FURAN-2-YLMETHYL)-AMINE](/StructureFile/ChemBookStructure7/GIF/CB9687949.gif)
626216-00-0
7 suppliers
$283.00/1g
Yield:626216-00-0 82.6%
Reaction Conditions:
Stage #1: 5-Methylfurfural;2-(3,4-dimethoxyphenyl)-ethylamine in ethanol; for 4 h;Heating / reflux;
Stage #2: with sodium tetrahydroborate in methanol at 20; for 16 h;
Stage #3: with water
Steps:
13 Preparation 13; Intermediate 1-5--Preparation of [[2- (3, 4-DIMETHOXYPHENYL) ETHYL]- (5-METHYLFU RAN-] [2-YLMETHYL)] amine
To a solution of 4.82 g (26. 6 mmol) of 2- (3, 4-dimethoxyphenyl) ethylamine and 3. [0 G] (27.2 [MMOL)] of [5-METHYLFURAN-2-CARBALDEHYDE] in 65 ml of EtOH, 18.3 g of molecular sieves (0.3 nm) were added and the mixture was refluxed for 4 hours. After this time the reaction mixture was cooled to room temperature and filtered. The solution obtained was concentrated in vacuo to obtain an oil. This oil was dissolved in 65 mi of MeOH arid 1. 01 g (26. 6 mmol) of NaBH4 were added in small portions, maintaining the temperature of the reaction at room temperature. The mixture was stirred at this temperature for 16 hours more. After this time the solvent was evaporated in vacuo and the residue was treated with 150 ml of water and extracted twice with ether. The organic layers were combined, washed with brine, dried over anhydrous MgSO4, filtered and evaporated to dryness to give 6.05 g (82.6%) of the title product as an oil. MS [M+1]+ : 276 ['H-NMR] [(CDC13)] : [S] 2.25 (s, 3H), 2.70-2. 95 (m, 4H), 3.75 (s, 2H), 3.85 (two singlets, 6H), 5.85 (m, 1H), 6.02 (m, 1H), 6.70-6. 85 [(M,] 3H).
References:
WO2004/840,2003,A2 Location in patent:Page 25-26