
2-Oxazolidinone, 5-(aMinoMethyl)-3-(3-fluoro-4-iodophenyl)-, (5S)- synthesis
- Product Name:2-Oxazolidinone, 5-(aMinoMethyl)-3-(3-fluoro-4-iodophenyl)-, (5S)-
- CAS Number:627543-03-7
- Molecular formula:C10H10FIN2O2
- Molecular Weight:336.1
![1H-Isoindole-1,3(2H)-dione, 2-[[(5S)-3-(3-fluoro-4-iodophenyl)-2-oxo-5-oxazolidinyl]Methyl]-](/CAS/GIF/724793-80-0.gif)
724793-80-0
4 suppliers
inquiry

627543-03-7
6 suppliers
inquiry
Yield:627543-03-7 90%
Reaction Conditions:
with hydrazine hydrate in ethanol; for 2 h;Reflux;
Steps:
General procedure-5: Formation of A5 aminomethyl analogs
General procedure: (1 mmol) of compound 5 was dissolved in ethanol (5 mL), then hydrazine monohydrate (5.0 equiv) was added and the resulting reaction mixture was heated to reflux and stirred for 2 hrs. Then the reaction mixture was cooled to room temperature before DDW (10 mL) was added. The aqueous solution was then extracted with DCM (3× 10 mL), and the combined organic extracts were washed with DDW (2× 10 mL) and saturated aqueous NaCl (10 mL), dried over MgSO4, and concentrated under vacuum.
References:
Spaulding, Andrew;Takrouri, Khuloud;Mahalingam, Pornachandran;Cleary, Dillon C.;Cooper, Harold D.;Zucchi, Paola;Tear, Westley;Koleva, Bilyana;Beuning, Penny J.;Hirsch, Elizabeth B.;Aggen, James B. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 23,p. 5310 - 5321] Location in patent:supporting information
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
34 suppliers
$208.00/250mg

627543-03-7
6 suppliers
inquiry
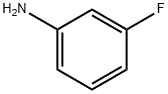
372-19-0
322 suppliers
$10.00/1g

627543-03-7
6 suppliers
inquiry
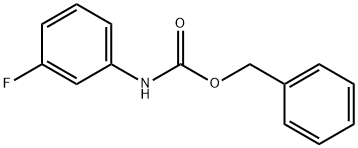
149524-47-0
47 suppliers
inquiry

627543-03-7
6 suppliers
inquiry
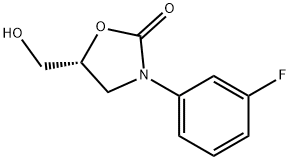
149524-42-5
95 suppliers
$34.00/100mg

627543-03-7
6 suppliers
inquiry