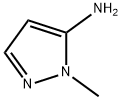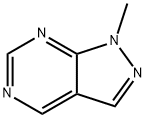
1H-Pyrazolo[3,4-d]pyrimidine, 1-methyl- (6CI,8CI,9CI) synthesis
- Product Name:1H-Pyrazolo[3,4-d]pyrimidine, 1-methyl- (6CI,8CI,9CI)
- CAS Number:6288-86-4
- Molecular formula:C6H6N4
- Molecular Weight:134.14
Yield:6288-86-4 93%
Reaction Conditions:
with trichlorophosphate in dichloromethane at 80 - 90;Inert atmosphere;
Steps:
1 Standard procedure for the preparation of pyrazolo[3,4-d]pyrimidine products 12a-e, 13a-e, and 14a-e
General procedure: The reliable procedure involved the treatment of N-1-phenyl-5-aminopyrazoles, N-1-phenyl-5-aminopyrazoles (1a-e, 1.0 equiv), N-1-(2-pyridinyl)-5-aminopyrazoles (4a-e 1.0 equiv), or N-1-(2-quinolinyl)-5-aminopyrazoles (5a-e, 1.0 equiv) with catalytic amount of POCl3 (~3 equiv) with formamide (2 mL) in CH2Cl2 solution at 80-90 °C within 0.5-1 h. When the reaction was completed, the reaction mixture was concentrated, added to saturate sodium bicarbonate (15 mL), and extracted with dichloromethane (15 mL). The organic extracts were dried over MgSO4, filtered, and concentrated under reduced pressure. The residues were purified by column chromatography on silica gel to give the corresponding pyrazolo[3,4-d]pyrimidine products 12a-e, 13a-e or 14a-e were obtained in 91-96%, 81-88%, or 82-91% yield, respectively.
References:
Chang, Chun-Hsi;Tsai, Henry J.;Huang, Yu-Ying;Lin, Hui-Yi;Wang, Li-Ya;Wu, Tian-Shung;Wong, Fung Fuh [Tetrahedron,2013,vol. 69,# 4,p. 1378 - 1386] Location in patent:supporting information
![4-Chloro-1-methyl-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/23000-43-3.gif)
23000-43-3
114 suppliers
$38.88/250mgs:
![1H-Pyrazolo[3,4-d]pyrimidine, 1-methyl- (6CI,8CI,9CI)](/CAS/GIF/6288-86-4.gif)
6288-86-4
16 suppliers
inquiry