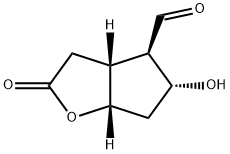
2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)- synthesis
- Product Name:2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-
- CAS Number:62961-72-2
- Molecular formula:C8H10O4
- Molecular Weight:170.16
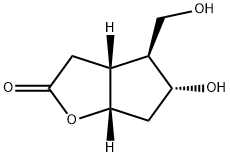
32233-40-2
362 suppliers
$9.00/250mg
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)
62961-72-2
0 suppliers
inquiry
Yield:62961-72-2 95%
Reaction Conditions:
with oxalyl dichloride in dichloromethane;dimethyl sulfoxide at -78; for 1.03333 h;Reagent/catalyst;Solvent;Temperature;
Steps:
1.1; 2.1; 3.1; 4.1; 5.1; 6.1; 7.1; 8.1; 9.1 (1) Synthesis of compound 2
At -78°C, a DCM solution (20 mL) of DMSO (3 ml, 42.2 mmol) was added dropwise to a DCM solution (20 mL) of oxalyl chloride (1.5 ml, 17.7 mmol).After stirring for 30 min, a solution of compound 1 (1.80 g, 10.5 mmol) in DCM (15 mL) was added dropwise to the above solution and stirred for 2 min. The reaction solution was kept at -78°C and stirred for 1 hour,Then TEA (12 mL, 86.0 mmol) was added dropwise to the above reaction liquid. After TEA was added dropwise, saturated NH4Cl solution (10 mL) was added to the reaction solution to quench the reaction. Then, the temperature of the reaction solution was raised to room temperature, and washed with saturated brine (3×20 mL),The organic phase was dried with anhydrous Na2SO4, filtered, concentrated, and purified by column chromatography to obtain compound 2 (1.7 g, 95%).
References:
CN111777538,2020,A Location in patent:Paragraph 0044-0047; 0060-0063; 0076-0079; 0092-0095; 0108
![(+)-(1R,5S)-2-Oxabicyclo[3.3.0]oct-6-en-3-one](/CAS/GIF/26054-46-6.gif)
26054-46-6
49 suppliers
inquiry
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)
62961-72-2
0 suppliers
inquiry
![7,7-Dichlorobicyclo[3.2.0]hept-2-en-6-one](/CAS/GIF/5307-99-3.gif)
5307-99-3
75 suppliers
$47.89/5g
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)
62961-72-2
0 suppliers
inquiry
542-92-7
91 suppliers
inquiry
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)
62961-72-2
0 suppliers
inquiry
![2H-Cyclopenta[b]furan-2-one, 5-(formyloxy)-4-[(formyloxy)methyl]hexahydro-, [3aR-(3aα,4α,5β,6aα)]- (9CI)](/CAS/20210305/GIF/130325-32-5.gif)
130325-32-5
0 suppliers
inquiry
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)
62961-72-2
0 suppliers
inquiry