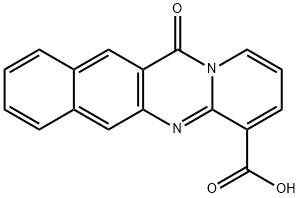
12-oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxylic acid synthesis
- Product Name:12-oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxylic acid
- CAS Number:63127-04-8
- Molecular formula:C17H10N2O3
- Molecular Weight:290.27
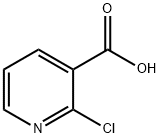
2942-59-8
782 suppliers
$5.00/25g
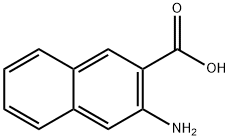
5959-52-4
207 suppliers
$42.00/1g
![12-oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxylic acid](/CAS2/GIF/63127-04-8.gif)
63127-04-8
15 suppliers
$122.00/100mg
Yield:63127-04-8 50.4%
Reaction Conditions:
with hydrogenchloride in ethanol at 80; for 72 h;
Steps:
2.1 4.2.1 12-Oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxylic acid (3)
A mixture of 39 3-amino-2-naphthoic acid (1) (4.00g, 21.36mmol), 40 2-chloronicotinic acid (2) (3.36g, 21.36mmol), and 41 hydrochloric acid (1.8mL, 60.00mmol) in 42 ethanol (150mL) was stirred at 80°C for 72h. After cooling, the reddish-orange suspension was filtered, washed with ethanol, and air-dried to afford 43 3 as a yellow-orange solid [26]. Yield: 2.12g, 50.4%. 1H NMR (400MHz, DMSO-d6) δ ppm 9.15 (s, 1H), 9.00 (dd, J=7.3, 1.4Hz, 1H), 8.60 (dd, J=6.9, 1.3Hz, 1H), 8.48 (s, 1H), 8.33 (d, J=8.4Hz, 1H), 8.18 (d, J=8.4Hz, 1H), 7.79-7.72 (m, 1H), 7.67-7.60 (m, 1H), 7.16 (t, J=7.1Hz, 1H).
References:
Zhang, Zhen-Lei;Zhao, Chun-Lai;Chen, Qian;Xu, Kai;Qiao, Xin;Xu, Jing-Yuan [European Journal of Medicinal Chemistry,2018,vol. 155,p. 434 - 444]