
4-(((4-Chlorophenyl)(4-(4-(trifluoromethoxy)phenyl)-thiazol-2-yl)amino)methyl)benzoic acid synthesis
- Product Name:4-(((4-Chlorophenyl)(4-(4-(trifluoromethoxy)phenyl)-thiazol-2-yl)amino)methyl)benzoic acid
- CAS Number:643012-93-5
- Molecular formula:C24H16ClF3N2O3S
- Molecular Weight:504.9087
![Benzoic acid, 4-[[(aminothioxomethyl)(4-chlorophenyl)amino]methyl]-](/CAS/20211123/GIF/643012-92-4.gif)
643012-92-4
0 suppliers
inquiry
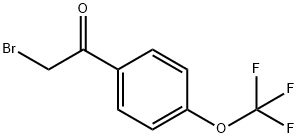
103962-10-3
132 suppliers
$6.00/250mg

643012-93-5
10 suppliers
$364.56/1g
Yield:643012-93-5 100%
Reaction Conditions:
Stage #1: 4-[1-(4-chlorophenyl)thioureidomethyl]benzoic acid;2-bromo-1-(4-(trifluoromethoxy)phenyl)ethanonewith acetic acid in DMF (N,N-dimethyl-formamide) at 20; for 16 h;
Stage #2: with ammonium chloride in DMF (N,N-dimethyl-formamide);water;ethyl acetate;
Steps:
429.B.3
Step 3, 4- ( { (4-chlorophenyl)- [4- (4-trifluoromethoxyphenyl) thiazol-2-yl] amino} methyl) benzoic acid : 4- [1- (4-Chlorophenyl) thioureidomethyl] benzoic acid (29 g, 88.8 mmol) was dissolved in DMF (300 mL) and acetic acid (40 mL) and 2'-bromo-4-trifluoromethoxyacetophenoe (25.1 g, 88.8 mmol) were added and the resulting mixture was stirred at room temperature for 16 hours. Ethyl acetate (500 mL) was added and the mixture was washed with water (2 x 500 mL), saturated aqueous sodium chloride (500 mL) and saturated aqueous ammonium chloride (500 mL). Drying (MgS04) and concentration in vacuo afforded 48 g (quant. ) of 4-({(4- chlorophenyl)- [4- (4-trifluoromethoxyphenyl) thiazol-2-yl] amino} methyl) benzoic acid as an oil. HPLC-MS (Method B): m/z: 505 (M+1); Rt: = 5.59 min.'H NMR (DMSO-d6) : 8 = 5.35 (2H, s), 7.36 (1H, s), 7.39 (2H, d), 7.47-7. 57 (6H, m), 7.90 (2H, d), 7.97 (2H, d), 12.7 (1H, bs).
References:
WO2004/2480,2004,A1 Location in patent:Page/Page column 139