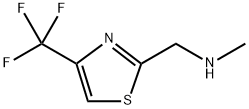
methyl({[4-(trifluoromethyl)-1,3-thiazol-2-yl]methyl})amine synthesis
- Product Name:methyl({[4-(trifluoromethyl)-1,3-thiazol-2-yl]methyl})amine
- CAS Number:644950-35-6
- Molecular formula:C6H7F3N2S
- Molecular Weight:196.19

354587-75-0
44 suppliers
$90.00/100mg

74-89-5
7 suppliers
$13.44/25ML
![methyl({[4-(trifluoromethyl)-1,3-thiazol-2-yl]methyl})amine](/CAS/20211123/GIF/644950-35-6.gif)
644950-35-6
3 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-(trifluoromethyl) thiazole-2-carbaldehyde;methylaminewith titanium(IV) isopropylate in methanol at 0; for 2 h;Inert atmosphere;
Stage #2: with sodium tetrahydroborate in methanol at 0 - 20;
Steps:
1.1.16
Ti(0'PR)4 (Commercial source: sigma-aldrich) (495 mg, 1.74 mmol) was added with stirring to MeNH2(Commercial source: sigma-aldrich) (2.0 M in MeOH, 2 ml, 4.02 mmol) under Ar. After 5 min. the aldehyde, 4-(trifluoromethyl)thiazole-2-carbaldehyde (245 mg, 1.34 mmol) was added, and the solution was stirred for 2 h. The reaction was cooled to 0° C and the NaBH4 (66 mg, 1.742 mmol) was added. The solution was stirred at 0° C to RT overnight. After quenching the reaction with water the mixture was filtered through celite to remove the white precipitate. Then MeOH was removed in vacuo. The residue was diluted with EtOAc. The resulting solution was washed with water (x3), brine (xl), and dried over Na2S04. The inorganics were filtered off, and the solvent was removed in vacuo to give the crude product. Purification via column chromatography yielded the pure N-methyl-l-(4- (trifluoromethyl)thiazol-2-yl)methanamine in 37% yield overall for 2 steps.
References:
WO2012/54510,2012,A1 Location in patent:Page/Page column 91
![[4-(trifluoromethyl)-1,3-thiazol-2-yl]methanol](/CAS/20180713/GIF/204319-69-7.gif)
204319-69-7
43 suppliers
$45.00/10mg
![methyl({[4-(trifluoromethyl)-1,3-thiazol-2-yl]methyl})amine](/CAS/20211123/GIF/644950-35-6.gif)
644950-35-6
3 suppliers
inquiry