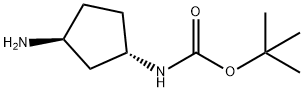
Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:645400-44-8
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28

A flask containing tert-butyl [(1S,3S)-3-aminocyclopentyl]carbamate (16.5 g, crude ~0.05 mol) and 1.7 g Pd-C (10% paste) in MeOH (300 mL) was exposed to a positive pressure of hydrogen gas (balloon) over weekend. The catalyst was filtered off and the mixture was concentrated to afford the title compound (9.5 g) as a thick colorless viscous oil. Tert-butyl [(1S,3S)-3-aminocyclopentyl]carbamate, Yield (9.5 g)
![Carbamic acid, [(1S,3S)-3-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopentyl]-, phenylmethyl ester (9CI)](/CAS/20210111/GIF/847417-31-6.gif)
847417-31-6
1 suppliers
inquiry
![Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/645400-44-8.gif)
645400-44-8
96 suppliers
$75.00/100mg
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol; under 1861.78 Torr; for 1 h;
Steps:
78.2 (1S,3S)-N-[2,2-difluoro-2-(4-methylphenyl)ethyl]-N'-1H-pyrazolo[3,4-d]pyrimidin-4-ylcyclopentane-1,3-diamine
Step 2: tert-butyl [(1S,3S)-3-aminocyclopentyl]carbamate A mixture of benzyl tert-butyl (1S,3S)-cyclopentane-1,3-diylbiscarbamate (2.02 g, 6.04 mmol) and 10% Pd/C (142 mg) in EtOH (200 mL) was shaken on a Parr apparatus under hydrogen (36 psi) for 1 hr. The reaction mixture was filtered through celite and concentrated to give the title compound (1.37 g) as an opaque paste.
References:
US2005/54658,2005,A1 Location in patent:Page/Page column 60
![Carbamic acid, N-[(1S,3S)-3-azidocyclopentyl]-, 1,1-dimethylethyl ester](/CAS/20211123/GIF/645400-43-7.gif)
645400-43-7
0 suppliers
inquiry
![Carbamic acid, [(1S,3S)-3-aminocyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/645400-44-8.gif)
645400-44-8
96 suppliers
$75.00/100mg