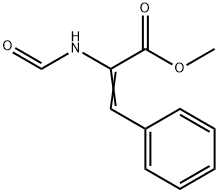
(Z)-2-FORMYLAMINO-3-PHENYL-ACRYLIC ACID METHYL ESTER synthesis
- Product Name:(Z)-2-FORMYLAMINO-3-PHENYL-ACRYLIC ACID METHYL ESTER
- CAS Number:64765-90-8
- Molecular formula:C11H11NO3
- Molecular Weight:205.21

39687-95-1
154 suppliers
$33.81/1g

100-52-7
944 suppliers
$20.37/250g

64765-90-8
11 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: isocyanoacetic acid methyl ester;benzaldehydewith sodium hydride in tetrahydrofuran;mineral oil at 20; for 2 h;
Stage #2: with acetic acid in tetrahydrofuran;mineral oil at 0;
Steps:
General procedure: A mixture of benzophenone (S1, 10.0 mmol) and methyl isocyanoacetate (S2, 10.0 mmol) in THF (10 ml) wasadded dropwise to a suspension of NaH (60% in oil) (12.0 mmol, 1.2 equiv) in THF (10.0 ml) at room temperature. After stirring for 2 h at room temperature, 10% AcOH was added to the mixture at 0 °C until there is no hydrogen release. The solvent was removed under reduced pressure and the residue was extracted with CH2Cl2 three times and the extract was washed with H2O, dried over Na2SO4 and concentrated under reduced pressure. Further recrystallization in MeOH afforded the product S3. THF (10.0 mL), NEt3 (40 mmol, 8.0 equiv) and S3 (5.0 mmol) were added to an oven-dried three necked flask under N2 atmosphere and cooled to 0°C. POCl3 (10.0 mmol, 2.0 equiv) was added dropwise and the mixture was stirred for 2 h at 0 °C after the addition S3 was completed. Then the mixture was quenched by sat. Na2CO3 and stirred for another 1 h. The mixture was extracted with CH2Cl2 three times, dried over Na2SO4 and concentracted under reduced pressure. The residue was purified by column chromatography on silica gel to give isocyanides.
References:
Xue, Dengqi;Xue, Yijie;Yu, Haihua;Shao, Liming [Synthesis,2019,vol. 51,# 4,p. 874 - 884] Location in patent:supporting information