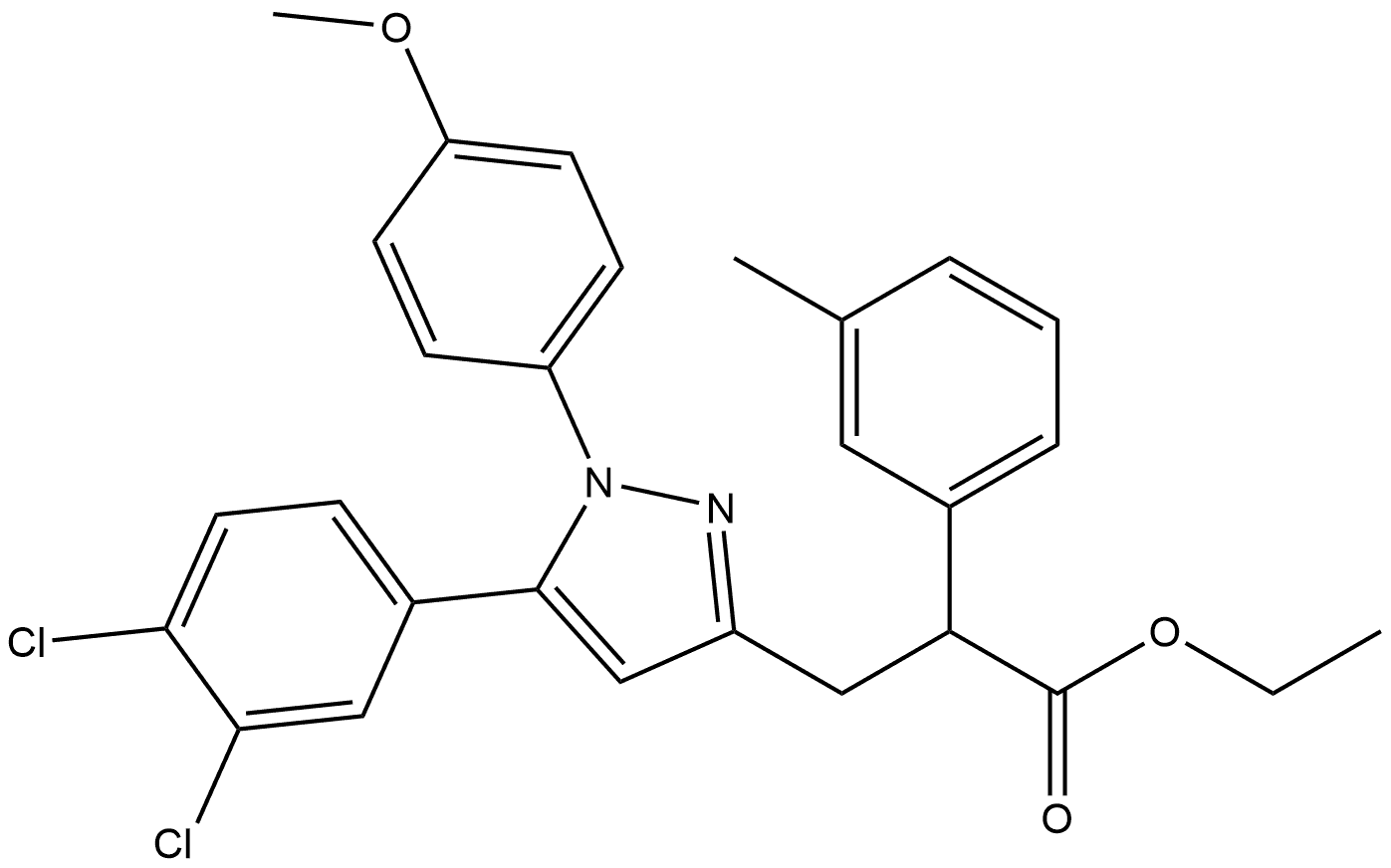
1H-Pyrazole-3-propanoic acid, 5-(3,4-dichlorophenyl)-1-(4-methoxyphenyl)-α-(3-methylphenyl)- synthesis
- Product Name:1H-Pyrazole-3-propanoic acid, 5-(3,4-dichlorophenyl)-1-(4-methoxyphenyl)-α-(3-methylphenyl)-
- CAS Number:648861-60-3
- Molecular formula:C26H22Cl2N2O3
- Molecular Weight:481.37
Yield:-
Reaction Conditions:
Stage #1: m-methylphenylacetic acidwith sodium hydride in DMF (N,N-dimethyl-formamide) at 0; for 1 h;
Stage #2: 3-bromomethyl-5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazole in DMF (N,N-dimethyl-formamide) at 20; for 18 h;
Stage #3: with lithium hydroxide in DMF (N,N-dimethyl-formamide);water at 20; for 18 h;
Steps:
2 SCHEME A.
IN] each of eight 10-mL test tubes, 60% NaH in mineral oil (18 mg, 0.45 [MMOL)] was suspended in 5 mL of [N,] [N-DIMETHYLFORMAMIDE] (DMF) at 0 [°C] under N2. Then, to each test tube, a unique phenyl-acetic acid ester [(A10)] was added, and the reaction mixtures were stirred for 1 h. Equal portions of the first such mixture were then loaded into the six wells of the first row of a 48-well Robbins block under N2, and equal portions of the next mixture were loaded into the six wells of the second row, and so on, until all eight reaction mixtures had been apportioned, and all forty-eight wells had been loaded. Then, 0.15 mmol of one of six different pyrazol bromides (A7, prepared analogously to the procedure described in Method 1) in 0.5 mL DMF was loaded into each of eight wells of the first of six orthogonal columns of the block, and 0.15 mmol of a second pyrazol bromide in 0.5 mL DMF was loaded into each of eight wells of the second column of the block, and so on, yielding a matrix of forty-eight unique reaction mixtures. After the block was shaken for 18 h at rt, 0.3 mL of 2 M aqueous [LIOH] was added to each well, and the block was shaken an additional 18 h at rt. The solutions were drained into the 48 wells of a Beckman microtiter collection plate, and the solvent was removed under reduced pressure. Each residue was dissolved in 1.5 mL of DMF and purified on a Gilson 215 prep-HPLC system (Method G; recoveries of 12-34 mg for the products, 16-44% yield, isolated as TFA salts). Example 2 [3- [5- (3, 4-DICHLORO-PHENYL)-1- (4-METHOXY-PHENYL)-1 H-PYRAZOL-3-YL]-2-M-TOLYL-] propionic acid. The title compound was prepared by Method 2: HPLC: [RT =10.] 46 (Method A), Rt= 4.81, 7.95 (Method C). MS (ES+): mass calculated for [C26H22CI2N203,] 480.10 ; [MLZ FOUND] 481.1 [M+H] +.'H NMR (400 MHz, [CDCI3)] : 7.31-7. 28 (m, 2H), 7.22 (d, J= 7.6 Hz, 1H), 7.21-7. 18 (m, 2H), 7.14-7. 08 (m, 3H), 6.89 (dd, J = [5. 3,] 2.0 Hz, 1 H), 6.85 (d, [J =] 8.5 Hz, 2H), 6.22 (s, 1 H), 4.13-4. 07 (m, 1H), 3.82 (s, 3H), 3.52 (dd, [J = 14.] 4,9. 1, Hz, 1H), 3.12 (dd, J= 10.1, 5.3 Hz, [1H),] 2.01 (s, [3H).]
References:
WO2004/7463,2004,A1 Location in patent:Page 138-139
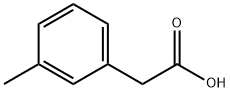
119540-36-2
0 suppliers
inquiry

648861-60-3
0 suppliers
inquiry
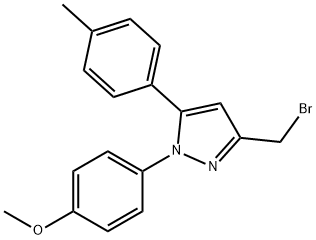
648869-66-3
0 suppliers
inquiry

648861-60-3
0 suppliers
inquiry
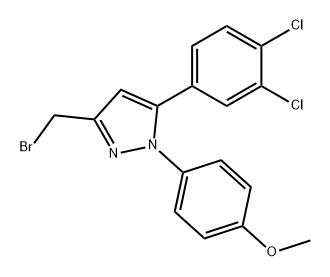
648869-73-2
0 suppliers
inquiry

648861-60-3
0 suppliers
inquiry
648869-25-4
0 suppliers
inquiry

648861-60-3
0 suppliers
inquiry