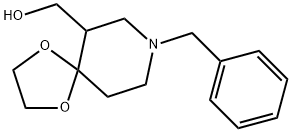
(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol synthesis
- Product Name:(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol
- CAS Number:64996-15-2
- Molecular formula:C15H21NO3
- Molecular Weight:263.33
![1,4-Dioxa-8-azaspiro[4.5]decane-6-carboxylic acid, 8-(phenylmethyl)-, ethyl ester](/CAS/20210305/GIF/955112-01-3.gif)
955112-01-3
0 suppliers
inquiry
![(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol](/CAS/20150408/GIF/64996-15-2.gif)
64996-15-2
28 suppliers
$28.60/50MG
Yield:64996-15-2 100%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;nitrogen;water;
Steps:
80.1.2 (80-1-2)
(80-1-2) 1-Benzyl-4,4-ethylenedioxy-3-piperidinemethanol In a stream of nitrogen, lithium aluminum hydride (702 mg) was carefully added to ice cooled dry tetrahydrofuran (100 ml). Into the resultant mixture was slowly added dropwise a solution of ethyl 1-benzyl-4,4-ethylenedioxy-3-piperidinecarboxylate (4.58 g) obtained above in tetrahydrofuran (30 ml). The resultant mixture was gradually heated and further stirred at room temperature overnight. Under ice cooling, water (0.7 ml), a 5 N aqueous solution (2.1 ml) of sodium hydroxide and further water (2.1 ml) were successively added to the reaction mixture carefully. Next, the resulting mixture was dried over anhydrous sodium sulfate and filtered through celite followed by concentration under reduced pressure. Thus the title compound (4.03 g) was obtained as a colorless oil (yield: 100%). 1H-NMR (400 MHz, CDCl3): δ(ppm) 1.67(1H, m), 1.92(1H, m), 2.01(1H, m), 2.43-2.66(3H, m), 2.70(1H, br-d), 3.49(2H, s), 3.77(1H, d, J=11.0Hz), 3.83(1H, d, J=11.0Hz), 3.96(4H, br-s), 7.23-7.33(5H, m).
References:
US2002/19531,2002,A1
![1,4-Dioxa-8-azaspiro[4.5]decane-6-carboxylic acid, 8-(phenylmethyl)-, methyl ester](/CAS/20200611/GIF/64996-14-1.gif)
64996-14-1
0 suppliers
inquiry
![(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol](/CAS/20150408/GIF/64996-15-2.gif)
64996-15-2
28 suppliers
$28.60/50MG
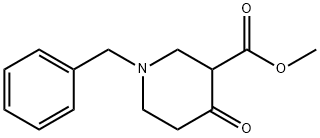
57611-47-9
79 suppliers
$47.00/1g
![(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol](/CAS/20150408/GIF/64996-15-2.gif)
64996-15-2
28 suppliers
$28.60/50MG
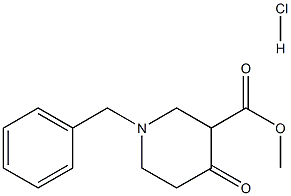
3939-01-3
132 suppliers
$5.00/1g
![(8-benzyl-1,4-dioxa-8-azaspiro[4.5]decan-6-yl)Methanol](/CAS/20150408/GIF/64996-15-2.gif)
64996-15-2
28 suppliers
$28.60/50MG