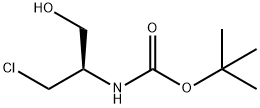
Carbamic acid, [(1R)-2-chloro-1-(hydroxymethyl)ethyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, [(1R)-2-chloro-1-(hydroxymethyl)ethyl]-, 1,1-dimethylethyl ester
- CAS Number:651035-90-4
- Molecular formula:C8H16ClNO3
- Molecular Weight:209.67

651035-84-6
26 suppliers
$145.00/0.25/ G
![Carbamic acid, [(1R)-2-chloro-1-(hydroxymethyl)ethyl]-, 1,1-dimethylethyl ester](/CAS/GIF/651035-90-4.gif)
651035-90-4
10 suppliers
inquiry
Yield:651035-90-4 100%
Reaction Conditions:
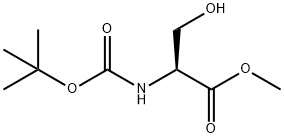
Stage #1: 2-(R)-tert-butoxycarbonylamino-3-chloro-propionic acid methyl esterwith lithium aluminium tetrahydride in tetrahydrofuran;ethanol at 0 - 20; for 4 h;Inert atmosphere;
Stage #2: with water;sodium hydrogencarbonate in tetrahydrofuran;ethanol;
Steps:
28.2
Step 2: ferf-butyl [(2R)-l-chloro-3-hydroxypropan-2-yl)carbamate (57)A solution of methyl N-(?r/-butoxycarbonyl)-3-chloro-L-alaninate (56, 2.13 g, 9.0 mmol) in ethanol (41 ml) stirred at 0 °C under N2 atmosphere was treated with a 2M solution of LiBH4 in THF (5 mL, 10 mmol) (slow addition). The reaction mixture was slowly warmed up to room temperature and further stirred for 4h, cooled to 0 °C, treated with saturated NaHC03 (5 mL) followed by acetone (5 mL), stirred for 10 minutes, diluted with DCM and washed with brine, dried over MgS04 filtered and concentrated to afford 1.94 (100% yield) of title compound as a white solid. MS : m/z= 232.0 (MNa+).
References:
WO2012/55034,2012,A1 Location in patent:Page/Page column 65-66
2766-43-0
297 suppliers
$10.00/1g
![Carbamic acid, [(1R)-2-chloro-1-(hydroxymethyl)ethyl]-, 1,1-dimethylethyl ester](/CAS/GIF/651035-90-4.gif)
651035-90-4
10 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G
![Carbamic acid, [(1R)-2-chloro-1-(hydroxymethyl)ethyl]-, 1,1-dimethylethyl ester](/CAS/GIF/651035-90-4.gif)
651035-90-4
10 suppliers
inquiry