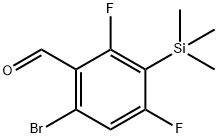
6-broMo-2,4-difluoro-3-(triMethylsilyl)benzaldehyde synthesis
- Product Name:6-broMo-2,4-difluoro-3-(triMethylsilyl)benzaldehyde
- CAS Number:651326-71-5
- Molecular formula:C10H11BrF2OSi
- Molecular Weight:293.18

184910-20-1
12 suppliers
inquiry

651326-71-5
9 suppliers
inquiry
Yield:651326-71-5 176 g
Reaction Conditions:
Stage #1: (4-bromo-2,6-difluoro-phenyl)-trimethyl-silanewith diisopropylamine;lithium diisopropyl amide in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: with acetic acid in tetrahydrofuran;hexane;water;N,N-dimethyl-formamide; for 2.5 h;
Steps:
2 Step 2: 6-Bromo-2,4-difluoro-3-(trimethylsilyl)benzaldehyde (C-XII-b)
At -78°C redistilled diisopropylamine (186 mL, 1.13 mol) was added dropwise to a solution of n- butyllithium in hexane (453 mL, 1.13 mol) in THF (1000 mL) cooled at -78°C within 10 min and stirring was continued for 1 h. Then, the freshly prepared LDA solution was added dropwise to a solution of (4-bromo-2,6-difluorophenyl)trimethylsilane (C-XII-a) (200 g, 0.754 mol) in THF (1000 mL) cooled to -78°C. The yellow solution was stirred for 1 h at -78°C before DMF (104 mL, 1.36 mol) was added dropwise within 5 min. The resulting yellow reaction mixture was stirred at -78°C for 1 h. Then, an acetic acid solution (188 mL) and water (800 mL) were added. The yellow suspension was stirred at RT for an additional 1.5 h. The two phases were separated and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, dried over anhydrous Na2SC>4, filtered and concentrated under reduced pressure affording the title product (176 g). UPLC-MS 1 : m/z 293.0/295.0 [M+H]+, tR = 1.25/1.38 min. 1H-NMR (600 MHz, DMSO-cfe) d ppm 10.13 (s, 1H), 7.61 (d, J = 8.3 Hz, 1 H), 0.35 (s, 9H).
References:
WO2021/186324,2021,A1 Location in patent:Page/Page column 122-123

461-96-1
478 suppliers
$6.00/10g

651326-71-5
9 suppliers
inquiry